Brain Tumor Res Treat.
2024 Jan;12(1):1-13. 10.14791/btrt.2023.0037.
Basics for Pediatric Brain Tumor Imaging: Techniques and Protocol Recommendations
- Affiliations
-
- 1Department of Radiology, Asan Medical Center, Seoul, Korea
- KMID: 2552334
- DOI: http://doi.org/10.14791/btrt.2023.0037
Abstract
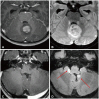
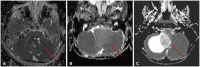
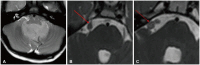
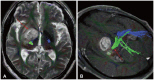
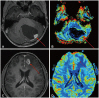
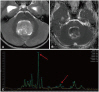
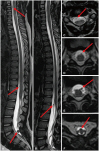
- This review provides an overview of the current state of pediatric brain tumor imaging, emphasizing the role of various imaging sequences and highlighting the advantages of standardizing protocols for pediatric brain tumor imaging in diagnosis and treatment response evaluation. Basic anatomical sequences such as pre- and post-contrast 3D T1-weighted, T2-weighted, fluid-attenuated inversion recovery, T2*-weighted, and diffusion-weighted imaging (DWI), are fundamental for assessing tumor location, extent, and characteristics. Advanced techniques like DWI, diffusion tensor imaging, perfusion imaging, magnetic resonance spectroscopy, and functional MRI offer insights into cellularity, vascularity, metabolism, and function. To enhance consistency and quality, standardized protocols for pediatric brain tumor imaging have been recommended by expert groups. Special considerations for pediatric patients, including the minimization of anesthesia exposure and gadolinium contrast agent usage, are essential to ensure patient safety and comfort. Staying up-to-date with diagnostic imaging techniques can contribute to improved communication, outcomes, and patient care in the field of pediatric neurooncology.
Keyword
Figure
Reference
-
1. Warren KE, Vezina G, Poussaint TY, Warmuth-Metz M, Chamberlain MC, Packer RJ, et al. Response assessment in medulloblastoma and leptomeningeal seeding tumors: recommendations from the Response Assessment in Pediatric Neuro-Oncology Committee. Neuro Oncol. 2018; 20:13–23. PMID: 28449033.
Article2. Fangusaro J, Witt O, Hernáiz Driever P, Bag AK, de Blank P, Kadom N, et al. Response assessment in paediatric low-grade glioma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) Working Group. Lancet Oncol. 2020; 21:e305–e316. PMID: 32502457.
Article3. Erker C, Tamrazi B, Poussaint TY, Mueller S, Mata-Mbemba D, Franceschi E, et al. Response assessment in paediatric high-grade glioma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) Working Group. Lancet Oncol. 2020; 21:e317–e329. PMID: 32502458.
Article4. Cooney TM, Cohen KJ, Guimaraes CV, Dhall G, Leach J, Massimino M, et al. Response assessment in diffuse intrinsic pontine glioma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) Working Group. Lancet Oncol. 2020; 21:e330–e336. PMID: 32502459.
Article5. Lindsay HB, Massimino M, Avula S, Stivaros S, Grundy R, Metrock K, et al. Response assessment in paediatric intracranial ependymoma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) Working Group. Lancet Oncol. 2022; 23:e393–e401. PMID: 35901835.
Article6. Hoffman LM, Jaimes C, Mankad K, Mirsky DM, Tamrazi B, Tinkle CL, et al. Response assessment in pediatric craniopharyngioma: recommendations from the response Assessment in Pediatric Neuro-Oncology (RAPNO) Working Group. Neuro Oncol. 2023; 25:224–233. PMID: 36124689.
Article7. Jaju A, Li Y, Dahmoush H, Gottardo NG, Laughlin S, Mirsky D, et al. Imaging of pediatric brain tumors: a COG diagnostic imaging committee/SPR oncology committee/ASPNR white paper. Pediatr Blood Cancer. 2023; 70(Suppl 4):e30147. PMID: 36519599.8. Kim DY, Kim PH, Jung AY, Choi JH, Cho YA, Yoon HM, et al. Neoplastic etiology and natural course of pituitary stalk thickening. J Clin Endocrinol Metab. 2022; 107:563–574. PMID: 34614160.
Article9. Do YA, Cho SJ, Choi BS, Baik SH, Bae YJ, Sunwoo L, et al. Predictive accuracy of T2-FLAIR mismatch sign for the IDH-mutant, 1p/19q noncodeleted low-grade glioma: an updated systematic review and meta-analysis. Neurooncol Adv. 2022; 4:vdac010. PMID: 35198981.
Article10. Wagner MW, Nobre L, Namdar K, Khalvati F, Tabori U, Hawkins C, et al. T2-FLAIR mismatch sign in pediatric low-grade glioma. AJNR Am J Neuroradiol. 2023; 44:841–845. PMID: 37348970.
Article11. Farnsworth RH, Lackmann M, Achen MG, Stacker SA. Vascular remodeling in cancer. Oncogene. 2014; 33:3496–3505. PMID: 23912450.
Article12. D’Arco F, Culleton S, De Cocker LJL, Mankad K, Davila J, Tamrazi B. Current concepts in radiologic assessment of pediatric brain tumors during treatment, part 1. Pediatr Radiol. 2018; 48:1833–1843. PMID: 29980859.
Article13. Villanueva-Meyer JE, Mabray MC, Cha S. Current clinical brain tumor imaging. Neurosurgery. 2017; 81:397–415. PMID: 28486641.
Article14. Cavallaro M, Coglitore A, Tessitore A, Galletta K, Frosina L, Cuffari A, et al. Three-dimensional constructive interference in steady state (3D CISS) imaging and clinical applications in brain pathology. Biomedicines. 2022; 10:2997. PMID: 36428564.
Article15. Pollice S, Capuano M, Scarabino T. Magnetic resonance technique. Scarabino T, Pollice S, editors. Imaging gliomas after treatment: a case-based atlas. Cham: Springer;2020. p. 41–45.16. Tamrazi B, Mankad K, Nelson M, D’Arco F. Current concepts and challenges in the radiologic assessment of brain tumors in children: part 2. Pediatr Radiol. 2018; 48:1844–1860. PMID: 30215111.
Article17. Chuang MT, Liu YS, Tsai YS, Chen YC, Wang CK. Differentiating radiation-induced necrosis from recurrent brain tumor using MR perfusion and spectroscopy: a meta-analysis. PLoS One. 2016; 11:e0141438. PMID: 26741961.
Article18. Nael K, Bauer AH, Hormigo A, Lemole M, Germano IM, Puig J, et al. Multiparametric MRI for differentiation of radiation necrosis from recurrent tumor in patients with treated glioblastoma. AJR Am J Roentgenol. 2018; 210:18–23. PMID: 28952810.
Article19. Artunduaga M, Liu CA, Morin CE, Serai SD, Udayasankar U, Greer MC, et al. Safety challenges related to the use of sedation and general anesthesia in pediatric patients undergoing magnetic resonance imaging examinations. Pediatr Radiol. 2021; 51:724–735. PMID: 33860861.
Article20. Tsai JW, Choi JJ, Ouaalam H, Murillo EA, Yeo KK, Vogelzang J, et al. Integrated response analysis of pediatric low-grade gliomas during and after targeted therapy treatment. Neurooncol Adv. 2023; 5:vdac182. PMID: 36926246.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Advanced Magnetic Resonance Imaging for Pediatric Brain Tumors: Current Imaging Techniques and Interpretation Algorithms
- Current Applications and Future Perspectives of Brain Tumor Imaging
- Emerging Techniques in Brain Tumor Imaging: What Radiologists Need to Know
- Advanced MRI for Pediatric Brain Tumors with Emphasis on Clinical Benefits
- Modern Brain Tumor Imaging