Korean J Radiol.
2017 Feb;18(1):132-151. 10.3348/kjr.2017.18.1.132.
Essential Items for Structured Reporting of Rectal Cancer MRI: 2016 Consensus Recommendation from the Korean Society of Abdominal Radiology
- KMID: 2468128
- DOI: http://doi.org/10.3348/kjr.2017.18.1.132
Abstract
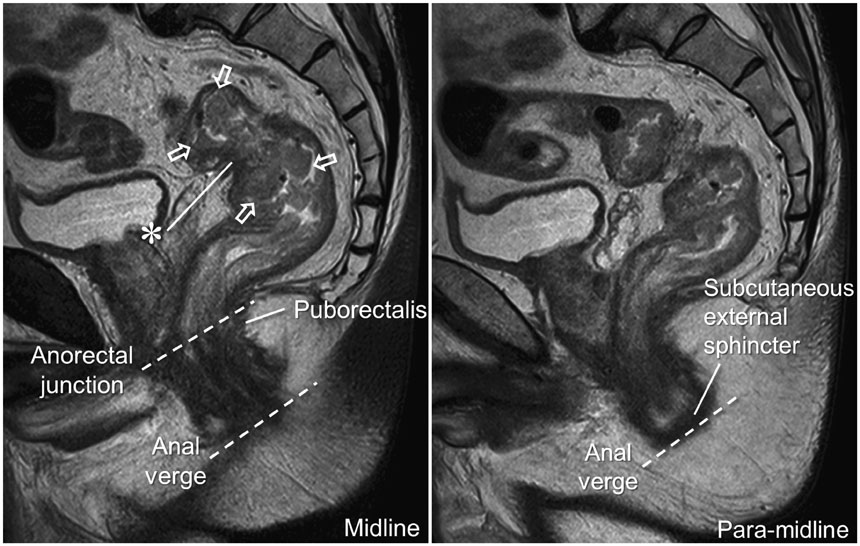
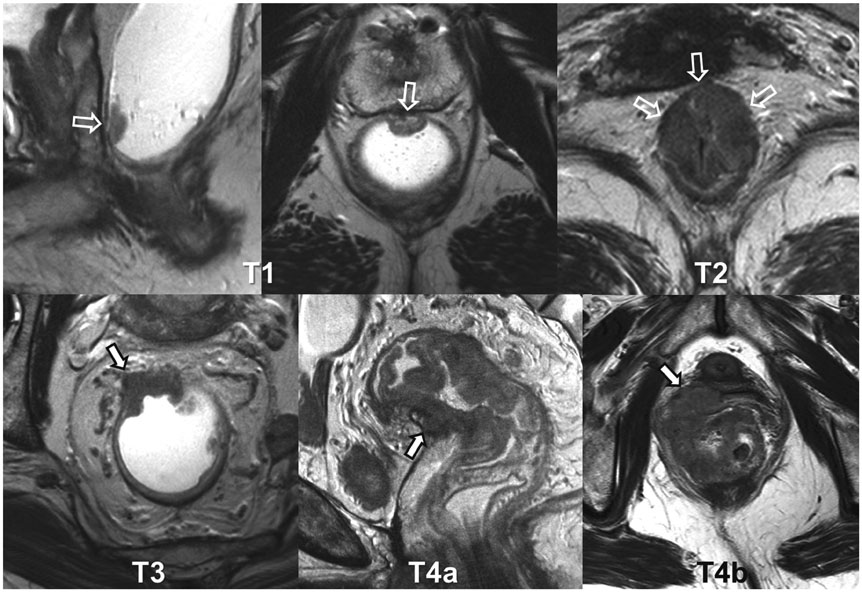
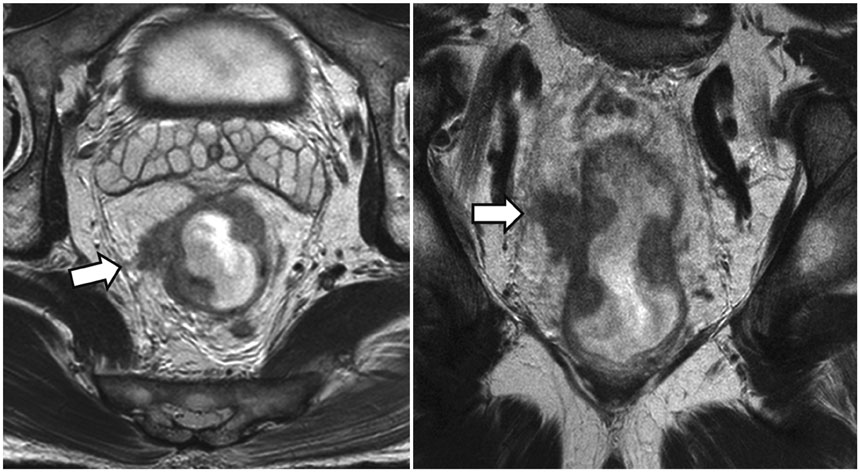
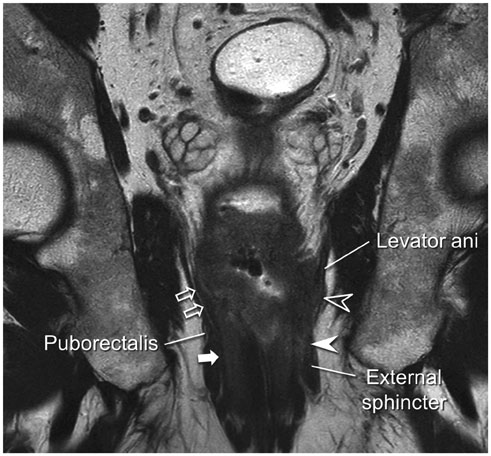
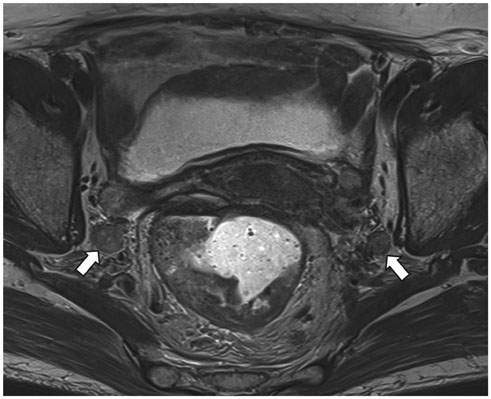
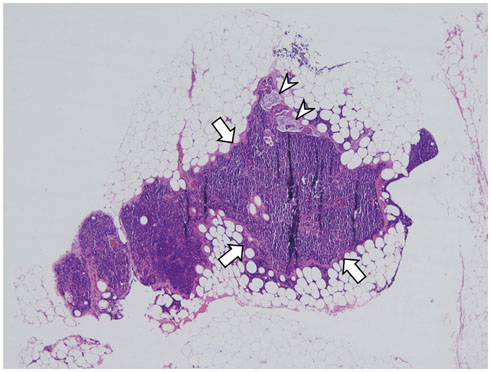
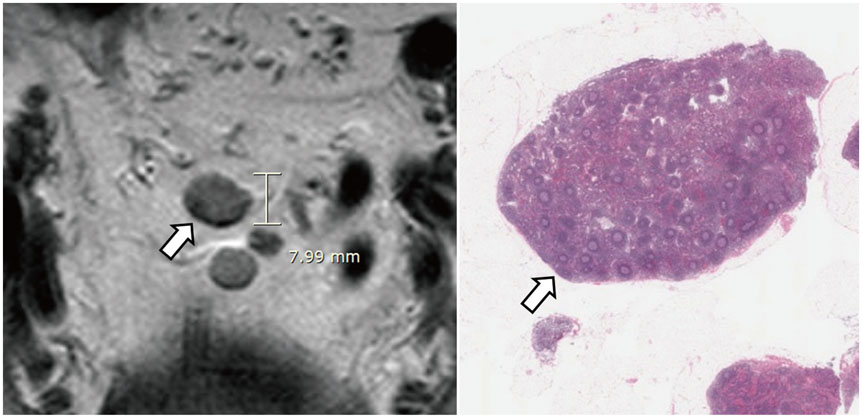
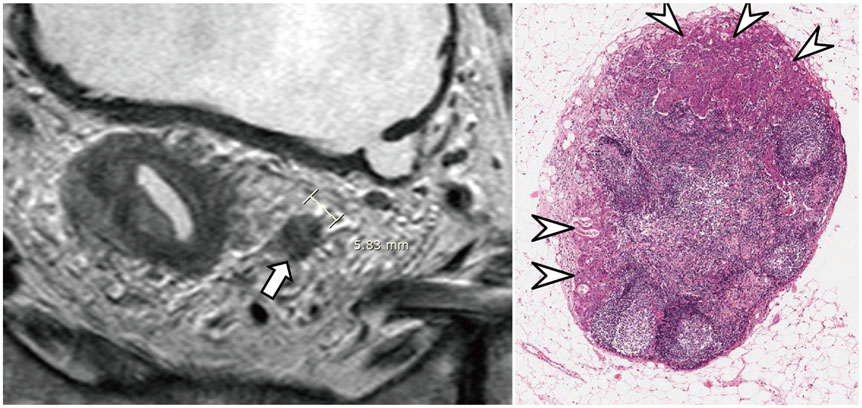
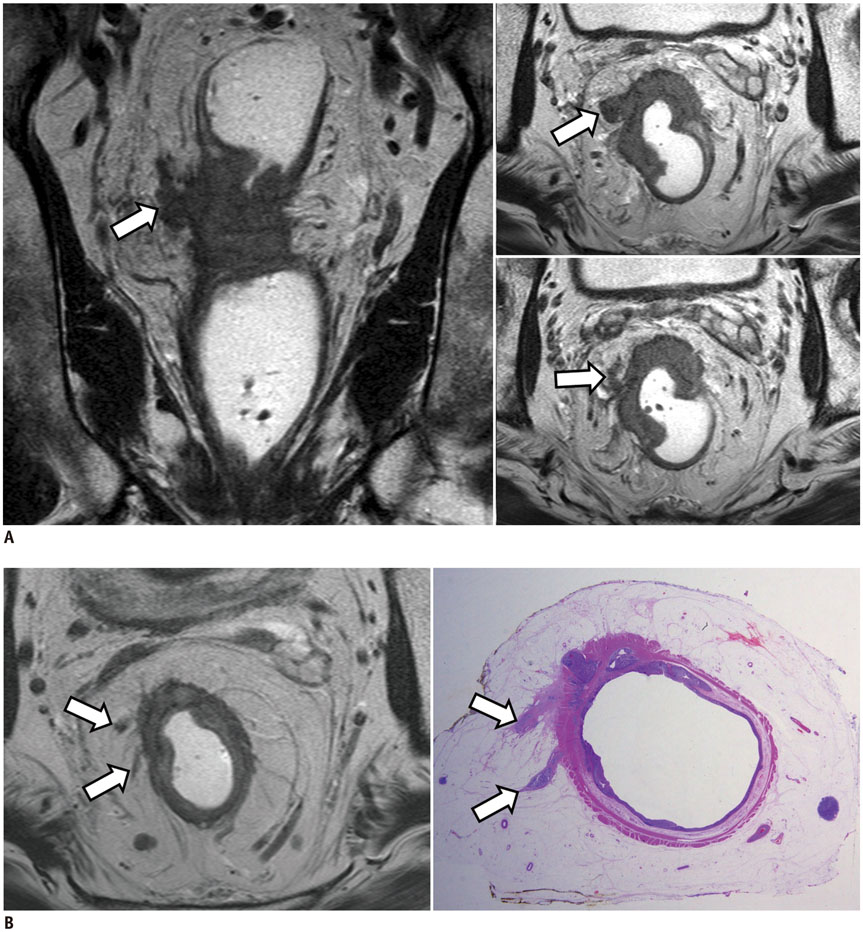
- High-resolution rectal MRI plays a crucial role in evaluating rectal cancer by providing multiple prognostic findings and imaging features that guide proper patient management. Quality reporting is critical for accurate effective communication of the information among multiple disciplines, for which a systematic structured approach is beneficial. Existing guides on reporting of rectal MRI are divergent on some issues, largely reflecting the differences in overall management of rectal cancer patients between the United States and Europe. The Korean Society of Abdominal Radiology (KSAR) study group for rectal cancer has developed an expert consensus recommendation regarding essential items for structured reporting of rectal cancer MRI using a modified Delphi method. This recommendation aims at presenting an up-to-date, evidence-based, practical, structured reporting template that can be readily adopted in daily clinical practice. In addition, a thorough explanation of the clinical and scientific rationale underlying the reporting items and their formats is provided. This KSAR recommendation may serve as a useful tool to help achieve more standardized optimal care for rectal cancer patients using rectal MRI.
Keyword
MeSH Terms
Figure
Reference
-
1. Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004; 351:1731–1740.2. Roh MS, Colangelo LH, O'Connell MJ, Yothers G, Deutsch M, Allegra CJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol. 2009; 27:5124–5130.3. Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001; 345:638–646.4. Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009; 373:811–820.5. Kennedy ED, Milot L, Fruitman M, Al-Sukhni E, Heine G, Schmocker S, et al. Development and implementation of a synoptic MRI report for preoperative staging of rectal cancer on a population-based level. Dis Colon Rectum. 2014; 57:700–708.6. Al-Sukhni E, Milot L, Fruitman M, Brown G, Schmocker S, Kennedy E. User's guide for the synoptic MRI report for rectal cancer. Web site. Accessed June 25, 2016. https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=133269.7. Al-Sukhni E, Milot L, Fruitman M, Brown G, Schmocker S, Kennedy E. MR Rectal Tumour. Web site. Accessed June 25, 2016. http://www.radreport.org/template/0000240.8. Taylor F, Mangat N, Swift IR, Brown G. Proforma-based reporting in rectal cancer. Cancer Imaging. 2010; 10 Spec no A:S142–S150.9. Beets-Tan RG, Lambregts DM, Maas M, Bipat S, Barbaro B, Caseiro-Alves F, et al. Magnetic resonance imaging for the clinical management of rectal cancer patients: recommendations from the 2012 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2013; 23:2522–2531.10. Tudyka V, Blomqvist L, Beets-Tan RG, Boelens PG, Valentini V, van de Velde CJ, et al. EURECCA consensus conference highlights about colon & rectal cancer multidisciplinary management: the radiology experts review. Eur J Surg Oncol. 2014; 40:469–475.11. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: rectal cancer version 2. Web site. Accessed June 25, 2016. https://www.nccn.org/professionals/physician_gls/PDF/rectal.pdf.12. van de Velde CJ, Boelens PG, Borras JM, Coebergh JW, Cervantes A, Blomqvist L, et al. EURECCA colorectal: multidisciplinary management: European consensus conference colon & rectum. Eur J Cancer. 2014; 50:1.e1–1.e34.13. American Joint Committee on Cancer. AJCC cancer staging manual. 7th ed. Chicago, IL: Springer;2010.14. Wittekind C, Compton CC, Brierley JR, Sobin L. TNM supplement: a commentary on uniform use. 4th ed. Oxford: Wiley-Blackwell;2012.15. Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. 7th ed. Oxford, UK: Wiley-Blackwell;2009.16. Salerno G, Sinnatamby C, Branagan G, Daniels IR, Heald RJ, Moran BJ. Defining the rectum: surgically, radiologically and anatomically. Colorectal Dis. 2006; 8:Suppl 3. 5–9.17. Schoellhammer HF, Gregorian AC, Sarkisyan GG, Petrie BA. How important is rigid proctosigmoidoscopy in localizing rectal cancer? Am J Surg. 2008; 196:904–908. discussion 908.18. Taylor FG, Swift RI, Blomqvist L, Brown G. A systematic approach to the interpretation of preoperative staging MRI for rectal cancer. AJR Am J Roentgenol. 2008; 191:1827–1835.19. Gowdra Halappa V, Corona Villalobos CP, Bonekamp S, Gearhart SL, Efron J, Herman J, et al. Rectal imaging: part 1, high-resolution MRI of carcinoma of the rectum at 3 T. AJR Am J Roentgenol. 2012; 199:W35–W42.20. Torkzad MR, Påhlman L, Glimelius B. Magnetic resonance imaging (MRI) in rectal cancer: a comprehensive review. Insights Imaging. 2010; 1:245–267.21. Corman ML, Bergamaschi RCM, Nicholls RJ, Fazio VW. Corman's colon and rectal surgery. 6th ed. Philadelphia: Lippincott Williams & Wilkins;2013.22. Jorge JM, Wexner SD. Anatomy and physiology of the rectum and anus. Eur J Surg. 1997; 163:723–731.23. Shihab OC, Moran BJ, Heald RJ, Quirke P, Brown G. MRI staging of low rectal cancer. Eur Radiol. 2009; 19:643–650.24. Gollub MJ, Maas M, Weiser M, Beets GL, Goodman K, Berkers L, et al. Recognition of the anterior peritoneal reflection at rectal MRI. AJR Am J Roentgenol. 2013; 200:97–101.25. Chang JS, Lee Y, Lim JS, Kim NK, Baik SH, Min BS, et al. The magnetic resonance imaging-based approach for identification of high-risk patients with upper rectal cancer. Ann Surg. 2014; 260:293–298.26. Merkel S, Mansmann U, Siassi M, Papadopoulos T, Hohenberger W, Hermanek P. The prognostic inhomogeneity in pT3 rectal carcinomas. Int J Colorectal Dis. 2001; 16:298–304.27. Beets-Tan RG, Beets GL, Vliegen RF, Kessels AG, Van Boven H, De Bruine A, et al. Accuracy of magnetic resonance imaging in prediction of tumour-free resection margin in rectal cancer surgery. Lancet. 2001; 357:497–504.28. Shepherd NA, Baxter KJ, Love SB. Influence of local peritoneal involvement on pelvic recurrence and prognosis in rectal cancer. J Clin Pathol. 1995; 48:849–855.29. Shin R, Jeong SY, Yoo HY, Park KJ, Heo SC, Kang GH, et al. Depth of mesorectal extension has prognostic significance in patients with T3 rectal cancer. Dis Colon Rectum. 2012; 55:1220–1228.30. Willett CG, Badizadegan K, Ancukiewicz M, Shellito PC. Prognostic factors in stage T3N0 rectal cancer: do all patients require postoperative pelvic irradiation and chemotherapy? Dis Colon Rectum. 1999; 42:167–173.31. Shirouzu K, Akagi Y, Fujita S, Ueno H, Takii Y, Komori K, et al. Clinical significance of the mesorectal extension of rectal cancer: a Japanese multi-institutional study. Ann Surg. 2011; 253:704–710.32. MERCURY Study Group. Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: results of the MERCURY study. Radiology. 2007; 243:132–139.33. Park SH. Degree of error of thin-section MR in measuring extramural depth of tumor invasion in patients with rectal cancer. Radiology. 2008; 246:647. author reply 647-648.34. Cho SH, Kim SH, Bae JH, Jang YJ, Kim HJ, Lee D, et al. Prognostic stratification by extramural depth of tumor invasion of primary rectal cancer based on the Radiological Society of North America proposal. AJR Am J Roentgenol. 2014; 202:1238–1244.35. Pedersen BG, Moran B, Brown G, Blomqvist L, Fenger-Grøn M, Laurberg S. Reproducibility of depth of extramural tumor spread and distance to circumferential resection margin at rectal MRI: enhancement of clinical guidelines for neoadjuvant therapy. AJR Am J Roentgenol. 2011; 197:1360–1366.36. Adam IJ, Mohamdee MO, Martin IG, Scott N, Finan PJ, Johnston D, et al. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet. 1994; 344:707–711.37. Quirke P, Durdey P, Dixon MF, Williams NS. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet. 1986; 2:996–999.38. MERCURY Study Group. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ. 2006; 333:779.39. Taylor FG, Quirke P, Heald RJ, Moran B, Blomqvist L, Swift I, et al. One millimetre is the safe cut-off for magnetic resonance imaging prediction of surgical margin status in rectal cancer. Br J Surg. 2011; 98:872–879.40. Taylor FG, Quirke P, Heald RJ, Moran BJ, Blomqvist L, Swift IR, et al. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY study. J Clin Oncol. 2014; 32:34–43.41. Engelen SM, Maas M, Lahaye MJ, Leijtens JW, van Berlo CL, Jansen RL, et al. Modern multidisciplinary treatment of rectal cancer based on staging with magnetic resonance imaging leads to excellent local control, but distant control remains a challenge. Eur J Cancer. 2013; 49:2311–2320.42. Xie H, Zhou X, Zhuo Z, Che S, Xie L, Fu W. Effectiveness of MRI for the assessment of mesorectal fascia involvement in patients with rectal cancer: a systematic review and meta-analysis. Dig Surg. 2014; 31:123–134.43. Shihab OC, Heald RJ, Rullier E, Brown G, Holm T, Quirke P, et al. Defining the surgical planes on MRI improves surgery for cancer of the low rectum. Lancet Oncol. 2009; 10:1207–1211.44. Salerno GV, Daniels IR, Moran BJ, Heald RJ, Thomas K, Brown G. Magnetic resonance imaging prediction of an involved surgical resection margin in low rectal cancer. Dis Colon Rectum. 2009; 52:632–639.45. Shihab OC, Quirke P, Heald RJ, Moran BJ, Brown G. Magnetic resonance imaging-detected lymph nodes close to the mesorectal fascia are rarely a cause of margin involvement after total mesorectal excision. Br J Surg. 2010; 97:1431–1436.46. Nagtegaal ID, Marijnen CA, Kranenbarg EK, van de Velde CJ, van Krieken JH. Pathology Review Committee. Cooperative Clinical Investigators. Circumferential margin involvement is still an important predictor of local recurrence in rectal carcinoma: not one millimeter but two millimeters is the limit. Am J Surg Pathol. 2002; 26:350–357.47. van Loenhout R, Zijta F, Lahaye M, Beets-Tan R, Smithuis R. Radiology assistant: rectal cancer-MR staging 2.0. Web site. 2016. Accessed June 25. http://www.radiologyassistant.nl/en/p56195b237699d/rectal-cancer-mr-staging-20.html.48. Battersby NJ, How P, Moran B, Stelzner S, West NP, Branagan G, et al. Prospective validation of a low rectal cancer magnetic resonance imaging staging system and development of a local recurrence risk stratification model: the mercury II study. Ann Surg. 2016; 263:751–760.49. Hermanek P, Merkel S, Fietkau R, Rödel C, Hohenberger W. Regional lymph node metastasis and locoregional recurrence of rectal carcinoma in the era of TME [corrected] surgery. Implications for treatment decisions. Int J Colorectal Dis. 2010; 25:359–368.50. Taylor FG, Quirke P, Heald RJ, Moran B, Blomqvist L, Swift I, et al. Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: a prospective, multicenter, European study. Ann Surg. 2011; 253:711–719.51. Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging--a meta-analysis. Radiology. 2004; 232:773–783.52. Lahaye MJ, Engelen SM, Nelemans PJ, Beets GL, van de Velde CJ, van Engelshoven JM, et al. Imaging for predicting the risk factors--the circumferential resection margin and nodal disease--of local recurrence in rectal cancer: a meta-analysis. Semin Ultrasound CT MR. 2005; 26:259–268.53. Al-Sukhni E, Milot L, Fruitman M, Beyene J, Victor JC, Schmocker S, et al. Diagnostic accuracy of MRI for assessment of T category, lymph node metastases, and circumferential resection margin involvement in patients with rectal cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2012; 19:2212–2223.54. Brown G, Richards CJ, Bourne MW, Newcombe RG, Radcliffe AG, Dallimore NS, et al. Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology. 2003; 227:371–377.55. Wang C, Zhou Z, Wang Z, Zheng Y, Zhao G, Yu Y, et al. Patterns of neoplastic foci and lymph node micrometastasis within the mesorectum. Langenbecks Arch Surg. 2005; 390:312–318.56. Kim JH, Beets GL, Kim MJ, Kessels AG, Beets-Tan RG. High-resolution MR imaging for nodal staging in rectal cancer: are there any criteria in addition to the size? Eur J Radiol. 2004; 52:78–83.57. Ogawa S, Hida J, Ike H, Kinugasa T, Ota M, Shinto E, et al. Selection of lymph node-positive cases based on perirectal and lateral pelvic lymph nodes using magnetic resonance imaging: study of the japanese society for cancer of the colon and rectum. Ann Surg Oncol. 2016; 23:1187–1194.58. Talbot IC, Ritchie S, Leighton MH, Hughes AO, Bussey HJ, Morson BC. The clinical significance of invasion of veins by rectal cancer. Br J Surg. 1980; 67:439–442.59. Chand M, Siddiqui MR, Swift I, Brown G. Systematic review of prognostic importance of extramural venous invasion in rectal cancer. World J Gastroenterol. 2016; 22:1721–1726.60. Smith NJ, Barbachano Y, Norman AR, Swift RI, Abulafi AM, Brown G. Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br J Surg. 2008; 95:229–236.61. Sohn B, Lim JS, Kim H, Myoung S, Choi J, Kim NK, et al. MRI-detected extramural vascular invasion is an independent prognostic factor for synchronous metastasis in patients with rectal cancer. Eur Radiol. 2015; 25:1347–1355.62. Bugg WG, Andreou AK, Biswas D, Toms AP, Williams SM. The prognostic significance of MRI-detected extramural venous invasion in rectal carcinoma. Clin Radiol. 2014; 69:619–623.63. Chand M, Bhangu A, Wotherspoon A, Stamp GW, Swift RI, Chau I, et al. EMVI-positive stage II rectal cancer has similar clinical outcomes as stage III disease following pre-operative chemoradiotherapy. Ann Oncol. 2014; 25:858–863.64. Smith NJ, Shihab O, Arnaout A, Swift RI, Brown G. MRI for detection of extramural vascular invasion in rectal cancer. AJR Am J Roentgenol. 2008; 191:1517–1522.65. Jhaveri KS, Hosseini-Nik H, Thipphavong S, Assarzadegan N, Menezes RJ, Kennedy ED, et al. MRI detection of extramural venous invasion in rectal cancer: correlation with histopathology using elastin stain. AJR Am J Roentgenol. 2016; 206:747–755.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Radiologic Report for Magnetic Resonance Imaging of Rectal Cancer before Treatment
- Imaging Diagnosis of Colorectal Cancer
- Standardized Imaging and Reporting for Thyroid Ultrasound: Korean Society of Thyroid Radiology Consensus Statement and Recommendation
- Interpretation of Rectal MRI after Neoadjuvant Treatment in Patients with Rectal Cancer
- Radiologic Evaluation and Structured Reporting Form for Extrahepatic Bile Duct Cancer: 2019 Consensus Recommendations from the Korean Society of Abdominal Radiology