J Clin Neurol.
2019 Oct;15(4):502-510. 10.3988/jcn.2019.15.4.502.
Language-Related White-Matter-Tract Deficits in Children with Benign Epilepsy with Centrotemporal Spikes: A Retrospective Study
- Affiliations
-
- 1Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Department of Diagnostic Radiology, Chonbuk National University Medical School, Jeonju, Korea.
- 3Department of Rehabilitation Medicine, Chonbuk National University Medical School, Jeonju, Korea.
- 4Department of Pediatrics, Chonbuk National University Medical School, Jeonju, Korea. sunjun@jbnu.ac.kr
- 5Research Institute of Clinical Medicine of Chonbuk National University, Jeonju, Korea.
- 6Biomedical Research Institute of Chonbuk National University Hospital, Chonbuk National University Medical School, Jeonju, Korea.
- KMID: 2467760
- DOI: http://doi.org/10.3988/jcn.2019.15.4.502
Abstract
- BACKGROUND AND PURPOSE
Benign epilepsy with centrotemporal spikes (BECTS) is one of the most common pediatric epilepsies, and it generally has a good prognosis. However, recent research has indicated that the epileptic activity of BECTS can cause cognitive defects such as language, visuospatial, and auditory verbal memory deficits. This study assessed language-delivery deficits in BECTS patients using diffusion-tensor magnetic resonance imaging (DTI).
METHODS
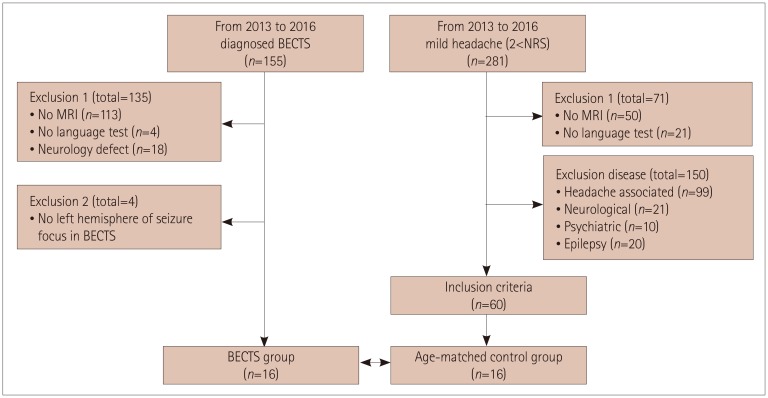
T1-weighted MRI, DTI, and language tests were conducted in 16 BECTS patients and 16 age-matched controls. DTI data were analyzed using the TRActs Constrained by Underlying Anatomy tool in FreeSurfer 5.3, and 18 major white-matter tracts were extracted, which included 4 language-related tracts: the inferior longitudinal fasciculus, superior longitudinal fasciculus-parietal terminations, superior longitudinal fasciculus-temporal terminations, and uncinate fasciculus (UNC). Language tests included the Korean version of the Receptive and Expressive Vocabulary Test, Test of Problem-Solving Abilities (TOPS), and the mean length of utterance in words.
RESULTS
The BECTS group exhibited decreased mean fractional anisotropy and increased mean radial diffusivity, with significant differences in both the superior longitudinal fasciculus and the left UNC (p<0.05), which are the language-related white-matter tracts in the dual-loop model. The TOPS language test scores were significantly lower in the BECTS group than in the control group (p<0.05).
CONCLUSIONS
It appears that BECTS patients can exhibit language deficits. Seizure activities of BECTS could alter DTI scalar values in the language-related white-matter tracts.
MeSH Terms
Figure
Reference
-
1. Northcott E, Connolly AM, Berroya A, Sabaz M, McIntyre J, Christie J, et al. The neuropsychological and language profile of children with benign rolandic epilepsy. Epilepsia. 2005; 46:924–930. PMID: 15946332.
Article2. Völkl-Kernstock S, Bauch-Prater S, Ponocny-Seliger E, Feucht M. Speech and school performance in children with benign partial epilepsy with centro-temporal spikes (BCECTS). Seizure. 2009; 18:320–326. PMID: 19249229.
Article3. Filippini M, Ardu E, Stefanelli S, Boni A, Gobbi G, Benso F. Neuropsychological profile in new-onset benign epilepsy with centrotemporal spikes (BECTS): focusing on executive functions. Epilepsy Behav. 2016; 54:71–79. PMID: 26667848.
Article4. Rijntjes M, Weiller C, Bormann T, Musso M. The dual loop model: its relation to language and other modalities. Front Evol Neurosci. 2012; 4:9. PMID: 22783188.
Article5. Démonet JF, Thierry G, Cardebat D. Renewal of the neurophysiology of language: functional neuroimaging. Physiol Rev. 2005; 85:49–95. PMID: 15618478.
Article6. Conturo TE, Lori NF, Cull TS, Akbudak E, Snyder AZ, Shimony JS, et al. Tracking neuronal fiber pathways in the living human brain. Proc Natl Acad Sci U S A. 1999; 96:10422–10427. PMID: 10468624.
Article7. Alexander AL, Lee JE, Lazar M, Boudos R, DuBray MB, Oakes TR, et al. Diffusion tensor imaging of the corpus callosum in Autism. Neuroimage. 2007; 34:61–73. PMID: 17023185.
Article8. Ameis SH, Lerch JP, Taylor MJ, Lee W, Viviano JD, Pipitone J, et al. A diffusion tensor imaging study in children with ADHD, autism spectrum disorder, OCD, and matched controls: distinct and non-distinct white matter disruption and dimensional brain-behavior relationships. Am J Psychiatry. 2016; 173:1213–1222. PMID: 27363509.
Article9. Friederici AD. Pathways to language: fiber tracts in the human brain. Trends Cogn Sci. 2009; 13:175–181. PMID: 19223226.
Article10. Sundgren PC, Dong Q, Gómez-Hassan D, Mukherji SK, Maly P, Welsh R. Diffusion tensor imaging of the brain: review of clinical applications. Neuroradiology. 2004; 46:339–350. PMID: 15103435.
Article11. Ciumas C, Saignavongs M, Ilski F, Herbillon V, Laurent A, Lothe A, et al. White matter development in children with benign childhood epilepsy with centro-temporal spikes. Brain. 2014; 137:1095–1106. PMID: 24598359.
Article12. Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001; 14:21–36. PMID: 11525331.
Article13. Afzali M, Soltanian-Zadeh H, Elisevich KV. Tract based spatial statistical analysis and voxel based morphometry of diffusion indices in temporal lobe epilepsy. Comput Biol Med. 2011; 41:1082–1091. PMID: 21616484.
Article14. Xiao F, Chen Q, Yu X, Tang Y, Luo C, Fang J, et al. Hemispheric lateralization of microstructural white matter abnormalities in children with active benign childhood epilepsy with centrotemporal spikes (BECTS): a preliminary DTI study. J Neurol Sci. 2014; 336:171–179. PMID: 24210075.
Article15. Cao W, Zhang Y, Hou C, Yang F, Gong J, Jiang S, et al. Abnormal asymmetry in benign epilepsy with unilateral and bilateral centrotemporal spikes: a combined fMRI and DTI study. Epilepsy Res. 2017; 135:56–63. PMID: 28623837.
Article16. Liamsuwan S, Grattan-Smith P, Fagan E, Bleasel A, Antony J. The value of partial sleep deprivation as a routine measure in pediatric electroencephalography. J Child Neurol. 2000; 15:26–29. PMID: 10641606.
Article17. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971; 9:97–113. PMID: 5146491.
Article18. Reuter M, Rosas HD, Fischl B. Highly accurate inverse consistent registration: a robust approach. Neuroimage. 2010; 53:1181–1196. PMID: 20637289.
Article19. Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002; 33:341–355. PMID: 11832223.20. Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000; 97:11050–11055. PMID: 10984517.
Article21. Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998; 17:87–97. PMID: 9617910.
Article22. Yendiki A, Panneck P, Srinivasan P, Stevens A, Zöllei L, Augustinack J, et al. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Front Neuroinform. 2011; 5:23. PMID: 22016733.
Article23. Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016; 125:1063–1078. PMID: 26481672.
Article24. Yendiki A, Koldewyn K, Kakunoori S, Kanwisher N, Fischl B. Spurious group differences due to head motion in a diffusion MRI study. Neuroimage. 2014; 88:79–90. PMID: 24269273.
Article25. Kim YT, Hong GH, Kim KH. Content and reliability analyses of the receptive and expressive vocabulary test (REVT). Commun Sci Disord. 2009; 14:34–45.26. Bae SY, Lim SS, Lee JH. Test of problem solving. Seoul: Seoul Community Rehabilitation Center;2000.27. Lee HJ, Kim YT. Measures of utterance length of normal language-delayed children. Commun Sci Disord. 1999; 4:1–14.28. Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn Reson Med. 2000; 44:259–268. PMID: 10918325.29. Madhavan KM, McQueeny T, Howe SR, Shear P, Szaflarski J. Superior longitudinal fasciculus and language functioning in healthy aging. Brain Res. 2014; 1562:11–22. PMID: 24680744.
Article30. Schmahmann JD, Pandya DN, Wang R, Dai G, D'Arceuil HE, De Crespigny AJ, et al. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007; 130:630–653. PMID: 17293361.
Article31. Yeterian EH, Pandya DN, Tomaiuolo F, Petrides M. The cortical connectivity of the prefrontal cortex in the monkey brain. Cortex. 2012; 48:58–81. PMID: 21481342.
Article32. Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS Jr, et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2005; 15:854–869. PMID: 15590909.
Article33. Catani M, Thiebaut de. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008; 44:1105–1132. PMID: 18619589.
Article34. Dubois J, Hertz-Pannier L, Cachia A, Mangin JF, Le Bihan D, Dehaene-Lambertz G. Structural asymmetries in the infant language and sensori-motor networks. Cereb Cortex. 2009; 19:414–423. PMID: 18562332.
Article35. Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix LR, Virta A, et al. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage. 2001; 13:1174–1185. PMID: 11352623.
Article36. Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002; 17:1429–1436. PMID: 12414282.
Article37. Takahashi M, Hackney DB, Zhang G, Wehrli SL, Wright AC, O'Brien WT, et al. Magnetic resonance microimaging of intraaxonal water diffusion in live excised lamprey spinal cord. Proc Natl Acad Sci U S A. 2002; 99:16192–16196. PMID: 12451179.
Article38. Chahine LM, Mikati MA. Benign pediatric localization-related epilepsies. Epileptic Disord. 2006; 8:243–258. PMID: 17150437.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Antiepileptic Drugs on Language Abilities in Benign Epilepsy of Childhood with Centrotemporal Spikes
- Children with Centrotemporal Spikes: Clinical and EEG Characteristics
- Cognitive and Behavioral Problems and the Effectiveness of Topiramate Once per Day in the Control of Benign Childhood Epilepsy with Centrotemporal Spikes
- A Case of a Coincidence of Rolandic and Childhood Absence Epilepsy
- Analysis of Interictal Epileptiform Discharges in the Benign Childhood Epilepsy with Centrotemporal Spikes: Prediction of Seizure Outcome