J Clin Neurol.
2019 Oct;15(4):496-501. 10.3988/jcn.2019.15.4.496.
Elevated Serum Uric Acid in Benign Convulsions with Mild Gastroenteritis in Children
- Affiliations
-
- 1Department of Pediatrics, Seoul National University Bundang Hospital, Seongnam, Korea. 5714@snubh.org
- 2Department of Pediatrics, St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Suwon, Korea.
- 3Pediatric Clinical Neuroscience Center, Department of Pediatrics, Seoul National University Children's Hospital, Seoul, Korea.
- 4Department of Biomedical Sciences, College of Medicine, Seoul National University, Seoul, Korea.
- 5Department of Pediatrics, SMG-SNU Boramae Medical Center, Seoul, Korea.
- KMID: 2467759
- DOI: http://doi.org/10.3988/jcn.2019.15.4.496
Abstract
- BACKGROUND AND PURPOSE
To identify whether serum uric acid levels are significantly higher in patients with benign convulsion associated with mild gastroenteritis (CwG) than in patients with acute gastroenteritis.
METHODS
This retrospective study compared the serum levels of uric acid between CwG, acute gastroenteritis, and febrile seizure after correcting for the varying degree of mild dehydration using serum HCO3â» levels. We also compared the serum uric acid levels between patients with CwG and febrile seizures in order to exclude the effect of seizures on uric acid.
RESULTS
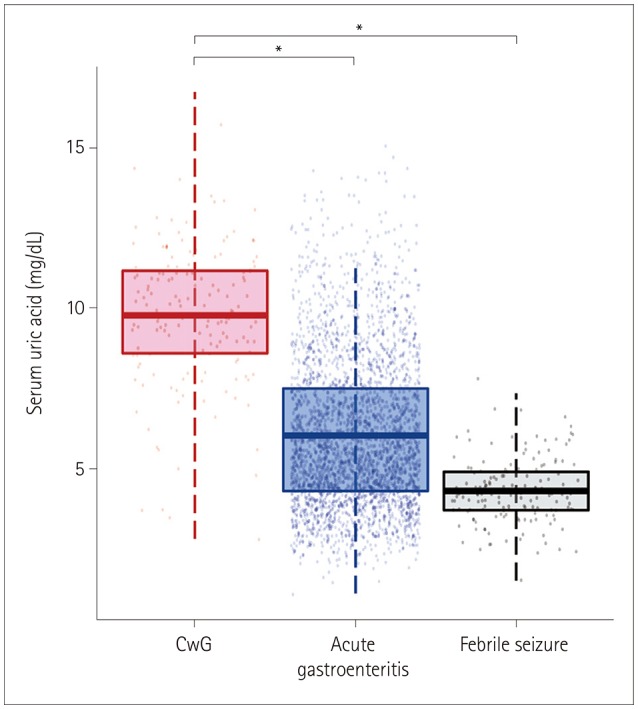
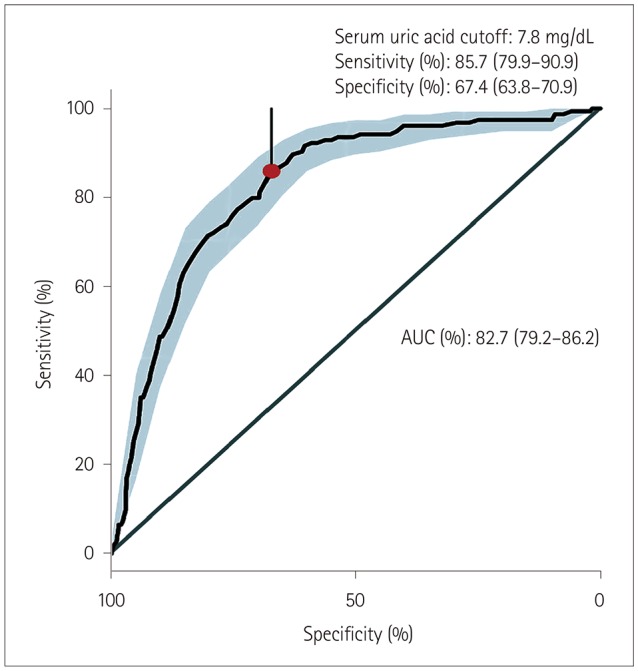
This study included 154 CwG patients (age range 0.73-3.19 years), 2,938 patients with acute gastroenteritis, and 154 patients with febrile seizure. The serum uric acid level was significantly higher in CwG patients than in patients with acute gastroenteritis [9.79±2.16 mg/dL vs. 6.04±2.3 mg/dL (mean±SD), p<0.001]. This difference was also significant after correcting for dehydration. The serum uric acid level was significantly higher in CwG patients than in dehydration-corrected acute gastroenteritis patients (9.79±2.16 mg/dL vs. 6.67±2.48 mg/dL, p<0.001). The serum uric acid level was not elevated in patients with febrile seizure.
CONCLUSIONS
We have confirmed that serum uric acid is elevated in CwG patients even after correcting for their dehydration status, and that this was not a postictal phenomenon. Highly elevated serum uric acid in CwG could be a useful clinical indicator of CwG in patients with acute gastroenteritis.
Keyword
MeSH Terms
Figure
Reference
-
1. Morooka K. Convulsions and mild diarrhea. Shonika. 1982; 23:134–137.2. DiFazio MP, Braun L, Freedman S, Hickey P. Rotavirus-induced seizures in childhood. J Child Neurol. 2007; 22:1367–1370. PMID: 18174553.
Article3. Narchi H. Benign afebrile cluster convulsions with gastroenteritis: an observational study. BMC Pediatr. 2004; 4:2. PMID: 15005806.
Article4. Verrotti A, Nanni G, Agostinelli S, Parisi P, Capovilla G, Beccaria F, et al. Benign convulsions associated with mild gastroenteritis: a multicenter clinical study. Epilepsy Res. 2011; 93:107–114. PMID: 21146369.
Article5. Castellazzi L, Principi N, Agostoni C, Esposito S. Benign convulsions in children with mild gastroenteritis. Eur J Paediatr Neurol. 2016; 20:690–695. PMID: 27292317.
Article6. Specchio N, Vigevano F. The spectrum of benign infantile seizures. Epilepsy Res. 2006; 70 Suppl 1:S156–S167. PMID: 16837167.
Article7. Tanabe T, Hara K, Kashiwagi M, Tamai H. Classification of benign infantile afebrile seizures. Epilepsy Res. 2006; 70 Suppl 1:S185–S189. PMID: 16814520.
Article8. Verrotti A, Moavero R, Vigevano F, Cantonetti L, Guerra A, Spezia E, et al. Long-term follow-up in children with benign convulsions associated with gastroenteritis. Eur J Paediatr Neurol. 2014; 18:572–577. PMID: 24780603.
Article9. Durá-Travé T, Yoldi-Petri ME, Molins-Castiella T, Souto-Hernández S, Aguilera-Albesa S. Infantile convulsions with mild gastroenteritis: epidemiological and clinical characteristics and outcome. Rev Neurol. 2010; 51:12–18. PMID: 20568063.10. Komori H, Wada M, Eto M, Oki H, Aida K, Fujimoto T. Benign convulsions with mild gastroenteritis: a report of 10 recent cases detailing clinical varieties. Brain Dev. 1995; 17:334–337. PMID: 8579220.
Article11. Chae SH, Rhee M, Kim YC, Kim SS. The relationship between serum uric acid level and benign convulsions with mild gastroenteritis. J Korean Child Neurol Soc. 2014; 22:191–194.12. Tsujita Y, Matsumoto H, Nakamura Y, Nonoyama S. Analysis of the blood and serum biochemistry findings in patients demonstrating convulsion with mild gastroenteritis. No To Hattatsu. 2011; 43:282–284. PMID: 21800691.13. World Health Organization. The treatment of diarrhoea: a manual for physicians and other senior health workers. Geneva: World Health Organization;2005.14. Kang B, Kim DH, Hong YJ, Son BK, Kim DW, Kwon YS. Comparison between febrile and afebrile seizures associated with mild rotavirus gastroenteritis. Seizure. 2013; 22:560–564. PMID: 23642407.
Article15. Kawano G, Oshige K, Syutou S, Koteda Y, Yokoyama T, Kim BG, et al. Benign infantile convulsions associated with mild gastroenteritis: a retrospective study of 39 cases including virological tests and efficacy of anticonvulsants. Brain Dev. 2007; 29:617–622. PMID: 17544607.
Article16. Li T, Hong S, Peng X, Cheng M, Jiang L. Benign infantile convulsions associated with mild gastroenteritis: an electroclinical study of 34 patients. Seizure. 2014; 23:16–19. PMID: 24125788.
Article17. Makki N, Hajj G, Schmidt GA. Seizure-induced acute urate nephropathy: case report and review. Chest. 2013; 144:666–669. PMID: 23918111.18. Thyrion L, Raedt R, Portelli J, Van Loo P, Wadman WJ, Glorieux G, et al. Uric acid is released in the brain during seizure activity and increases severity of seizures in a mouse model for acute limbic seizures. Exp Neurol. 2016; 277:244–251. PMID: 26774005.
Article19. Steiner MJ, DeWalt DA, Byerley JS. Is this child dehydrated? JAMA. 2004; 291:2746–2754. PMID: 15187057.
Article20. Vega RM, Avner JR. A prospective study of the usefulness of clinical and laboratory parameters for predicting percentage of dehydration in children. Pediatr Emerg Care. 1997; 13:179–182. PMID: 9220501.
Article21. Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011; 12:77. PMID: 21414208.
Article22. Kuge R, Morikawa Y, Hasegawa Y. Uric acid and dehydration in children with gastroenteritis. Pediatr Int. 2017; 59:1151–1156. PMID: 28714223.
Article23. Tam RK, Wong H, Plint A, Lepage N, Filler G. Comparison of clinical and biochemical markers of dehydration with the clinical dehydration scale in children: a case comparison trial. BMC Pediatr. 2014; 14:149. PMID: 24935348.
Article24. Warren DJ, Leitch AG, Leggett RJ. Hyperuricaemic acute renal failure after epileptic seizures. Lancet. 1975; 2:385–387. PMID: 51191.25. Spitsin S, Markowitz CE, Zimmerman V, Koprowski H, Hooper DC. Modulation of serum uric acid levels by inosine in patients with multiple sclerosis does not affect blood pressure. J Hum Hypertens. 2010; 24:359–362. PMID: 19865105.
Article26. Andreadou E, Nikolaou C, Gournaras F, Rentzos M, Boufidou F, Tsoutsou A, et al. Serum uric acid levels in patients with Parkinson's disease: their relationship to treatment and disease duration. Clin Neurol Neurosurg. 2009; 111:724–728. PMID: 19632030.
Article27. Alonso A, Rodríguez LA, Logroscino G, Hernán MA. Gout and risk of Parkinson disease: a prospective study. Neurology. 2007; 69:1696–1700. PMID: 17954784.
Article28. Hamed SA, Hamed EA, Hamdy R, Nabeshima T. Vascular risk factors and oxidative stress as independent predictors of asymptomatic atherosclerosis in adult patients with epilepsy. Epilepsy Res. 2007; 74:183–192. PMID: 17448640.
Article29. Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, Iyer AM, et al. Tolllike receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med. 2010; 16:413–419. PMID: 20348922.
Article30. Matsuo H, Ichida K, Takada T, Nakayama A, Nakashima H, Nakamura T, et al. Common dysfunctional variants in ABCG2 are a major cause of early-onset gout. Sci Rep. 2013; 3:2014. PMID: 23774753.
Article31. Matsuo H, Chiba T, Nagamori S, Nakayama A, Domoto H, Phetdee K, et al. Mutations in glucose transporter 9 gene SLC2A9 cause renal hypouricemia. Am J Hum Genet. 2008; 83:744–751. PMID: 19026395.
Article32. Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH, et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 2002; 417:447–452. PMID: 12024214.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Serum Uric Acid as a Predictive Factor for Rotaviral or Noroviral Benign Convulsions with Mild Gastroenteritis
- Update on benign convulsions with mild gastroenteritis
- Clinical Usefulness of Serum Uric Acid in Gastroenteritis Patients with Dehydration
- Can Serum Sodium Level Affect Seizure Features of Benign Convulsions with Mild Gastroenteritis?
- Erratum to: Elevated Serum Uric Acid in Benign Convulsions with Mild Gastroenteritis in Children