J Clin Neurol.
2019 Oct;15(4):448-453. 10.3988/jcn.2019.15.4.448.
Subcortical Volume Changes in Migraine with Aura
- Affiliations
-
- 1Laboratory for Advanced Analysis of Neuroimages, Faculty of Physical Chemistry, University of Belgrade, Belgrade, Serbia. ip7med@yahoo.com
- 2Faculty of Medicine, University of Belgrade, Belgrade, Serbia.
- 3Center for headaches, Neurology Clinic, Clinical Center of Serbia, Belgrade, Serbia.
- KMID: 2467752
- DOI: http://doi.org/10.3988/jcn.2019.15.4.448
Abstract
- BACKGROUND AND PURPOSE
Various features of the cerebral cortex and white matter have been extensively investigated in migraine with aura (MwA), but the morphological characteristics of subcortical structures have been largely neglected. The aim of this study was to identify possible differences in subcortical structures between MwA patients and healthy subjects (HS), and also to determine the correlations between the characteristics of migraine aura and the volumes of subcortical structures.
METHODS
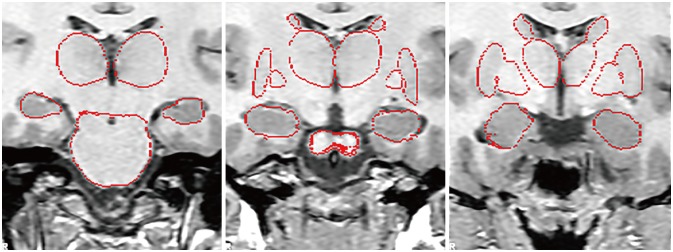
Thirty-two MwA patients and 32 HS matched by sex and age were analyzed in this study. Regional subcortical brain volumes were automatically calculated using the FSL/FMRIB Image Registration and Segmentation Tool software (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Glossary). A general linear model analysis was used to investigate differences in the volume of subcortical structures between the MwA patients and HS. A partial correlation test was used to assess correlations between the volume of subcortical structures and characteristics of MwA.
RESULTS
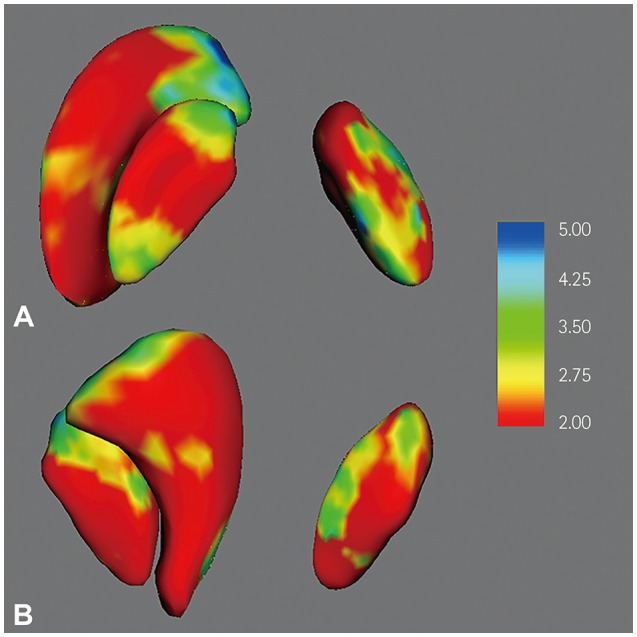
The volumes of the right globus pallidus, left globus pallidus, and left putamen were significantly smaller in MwA patients than in HS (mean±SD): 1,427±135 mm³ vs. 1,557±136 mm³ (p<0.001), 1,436±126 mm³ vs. 1,550±139 mm³ (p=0.001), and 4,235±437 mm³ vs. 4,522±412 mm³ (p=0.006), respectively. There were no significant relationships between subcortical structures and clinical parameters.
CONCLUSIONS
These findings suggest that both the globus pallidi and left putamen play significant roles in the pathophysiology of the MwA. Future studies should determine the cause-and-effect relationships, since these could not be discriminated in this study due to its cross-sectional design.
Keyword
MeSH Terms
Figure
Reference
-
1. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition. Cephalalgia. 2018; 38:1–211.2. Hadjikhani N, Sanchez Del Rio M, Wu O, Schwartz D, Bakker D, Fischl B, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001; 98:4687–4692. PMID: 11287655.
Article3. Rogawski MA. Common pathophysiologic mechanisms in migraine and epilepsy. Arch Neurol. 2008; 65:709–714. PMID: 18541791.
Article4. Martens-Mantai T, Speckmann EJ, Gorji A. Propagation of cortical spreading depression into the hippocampus: the role of the entorhinal cortex. Synapse. 2014; 68:574–584. PMID: 25049108.
Article5. Kim JH, Suh SI, Seol HY, Oh K, Seo WK, Yu SW, et al. Regional grey matter changes in patients with migraine: a voxel-based morphometry study. Cephalalgia. 2008; 28:598–604. PMID: 18422725.
Article6. DaSilva AF, Granziera C, Snyder J, Hadjikhani N. Thickening in the somatosensory cortex of patients with migraine. Neurology. 2007; 69:1990–1995. PMID: 18025393.
Article7. Bashir A, Lipton RB, Ashina S, Ashina M. Migraine and structural changes in the brain: a systematic review and meta-analysis. Neurology. 2013; 81:1260–1268. PMID: 23986301.
Article8. Chong CD, Schwedt TJ, Dodick DW. Migraine: what imaging reveals. Curr Neurol Neurosci Rep. 2016; 16:64. PMID: 27181270.
Article9. Maleki N, Becerra L, Nutile L, Pendse G, Brawn J, Bigal M, et al. Migraine attacks the basal ganglia. Mol Pain. 2011; 7:71. PMID: 21936901.
Article10. Yuan K, Zhao L, Cheng P, Yu D, Zhao L, Dong T, et al. Altered structure and resting-state functional connectivity of the basal ganglia in migraine patients without aura. J Pain. 2013; 14:836–844. PMID: 23669074.
Article11. Liu J, Lan L, Li G, Yan X, Nan J, Xiong S, et al. Migraine-related gray matter and white matter changes at a 1-year follow-up evaluation. J Pain. 2013; 14:1703–1708. PMID: 24290450.12. Magon S, May A, Stankewitz A, Goadsby PJ, Tso AR, Ashina M, et al. Morphological abnormalities of thalamic subnuclei in migraine: a multicenter MRI study at 3 tesla. J Neurosci. 2015; 35:13800–13806. PMID: 26446230.
Article13. Chong CD, Dumkrieger GM, Schwedt TJ. Structural co-variance patterns in migraine: a cross-sectional study exploring the role of the hippocampus. Headache. 2017; 57:1522–1531. PMID: 28976002.
Article14. Husøy AK, Pintzka C, Eikenes L, Håberg AK, Hagen K, Linde M, et al. Volume and shape of subcortical grey matter structures related to headache: a cross-sectional population-based imaging study in the Nord-Trøndelag Health Study. Cephalalgia. 2019; 39:173–184. PMID: 29848110.
Article15. Petrusic I, Zidverc-Trajkovic J, Podgorac A, Sternic N. Underestimated phenomena: higher cortical dysfunctions during migraine aura. Cephalalgia. 2013; 33:861–867. PMID: 23430982.
Article16. Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011; 56:907–922. PMID: 21352927.
Article17. Sameti M, Smith S, Patenaude B, Fein G. Subcortical volumes in longterm abstinent alcoholics: associations with psychiatric comorbidity. Alcohol Clin Exp Res. 2011; 35:1067–1080. PMID: 21332530.
Article18. Amann M, Andělová M, Pfister A, Mueller-Lenke N, Traud S, Reinhardt J, et al. Subcortical brain segmentation of two dimensional T1-weighted data sets with FMRIB's Integrated Registration and Segmentation Tool (FIRST). Neuroimage Clin. 2014; 7:43–52. PMID: 25610766.
Article19. Nugent AC, Luckenbaugh DA, Wood SE, Bogers W, Zarate CA Jr, Drevets WC. Automated subcortical segmentation using FIRST: test-retest reliability, interscanner reliability, and comparison to manual segmentation. Hum Brain Mapp. 2013; 34:2313–2329. PMID: 22815187.
Article20. Kita H. Globus pallidus external segment. Prog Brain Res. 2007; 160:111–133. PMID: 17499111.
Article21. Hoover JE, Strick PL. Multiple output channels in the basal ganglia. Science. 1993; 259:819–821. PMID: 7679223.
Article22. Ni Z, Kim SJ, Phielipp N, Ghosh S, Udupa K, Gunraj CA, et al. Pallidal deep brain stimulation modulates cortical excitability and plasticity. Ann Neurol. 2018; 83:352–362. PMID: 29369401.
Article23. Mao CP, Bai ZL, Zhang XN, Zhang QJ, Zhang L. Abnormal subcortical brain morphology in patients with knee osteoarthritis: a crosssectional study. Front Aging Neurosci. 2016; 8:3. PMID: 26834629.
Article24. Luchtmann M, Steinecke Y, Baecke S, Lützkendorf R, Bernarding J, Kohl J, et al. Structural brain alterations in patients with lumbar disc herniation: a preliminary study. PLoS One. 2014; 9:e90816. PMID: 24595036.
Article25. Mao C, Wei L, Zhang Q, Liao X, Yang X, Zhang M. Differences in brain structure in patients with distinct sites of chronic pain: a voxel-based morphometric analysis. Neural Regen Res. 2013; 8:2981–2990. PMID: 25206618.26. Wu Q, Inman RD, Davis KD. Neuropathic pain in ankylosing spondylitis: a psychophysics and brain imaging study. Arthritis Rheum. 2013; 65:1494–1503. PMID: 23460087.
Article27. Schmidt-Wilcke T, Luerding R, Weigand T, Jürgens T, Schuierer G, Leinisch E, et al. Striatal grey matter increase in patients suffering from fibromyalgia--a voxel-based morphometry study. Pain. 2007; 132(Suppl 1):S109–S116. PMID: 17587497.28. Messina R, Rocca MA, Colombo B, Valsasina P, Horsfield MA, Copetti M, et al. Cortical abnormalities in patients with migraine: a surface-based analysis. Radiology. 2013; 268:170–180. PMID: 23533286.
Article29. Petrusic I, Dakovic M, Kacar K, Zidverc-Trajkovic J. Migraine with aura: surface-based analysis of cerebral cortex with magnetic resonance imaging. Korean J Radiol. 2018; 19:767–776. PMID: 29962883.30. Du H, Zhang Y, Xie B, Wu N, Wu G, Wang J, et al. Regional atrophy of the basal ganglia and thalamus in idiopathic generalized epilepsy. J Magn Reson Imaging. 2011; 33:817–821. PMID: 21448945.
Article31. Peng SJ, Harnod T, Tsai JZ, Ker MD, Chiou JC, Chiueh H, et al. Evaluation of subcortical grey matter abnormalities in patients with MRInegative cortical epilepsy determined through structural and tensor magnetic resonance imaging. BMC Neurol. 2014; 14:104. PMID: 24885823.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Characteristics of Migraine with Aura in Korean: a Clinic Based Study
- White Matter Abnormalities of Migraine and Tension Type Headache in Young Patients Without Vascular Risk Factors
- A study on the therapeutic effects of Topiramate according to the types of migraine
- A Case of Successful Treatment During Migraine Aura Using Isometheptene Compound
- Typical Aura without Headache Presenting Intermittent Transient Visual Symptom