Anat Cell Biol.
2019 Dec;52(4):511-517. 10.5115/acb.19.114.
Regulation of estrous cycle by Cynodon dactylon in letrozole induced polycystic ovarian syndrome in Wistars albino rats
- Affiliations
-
- 1Department of Anatomy, Sri Lakshmi Narayana Institute of Medical Sciences, Puducherry, India. anandaraman2006@gmail.com
- 2Bharath Institute of Higher Education and Research, Chennai, India.
- KMID: 2466705
- DOI: http://doi.org/10.5115/acb.19.114
Abstract
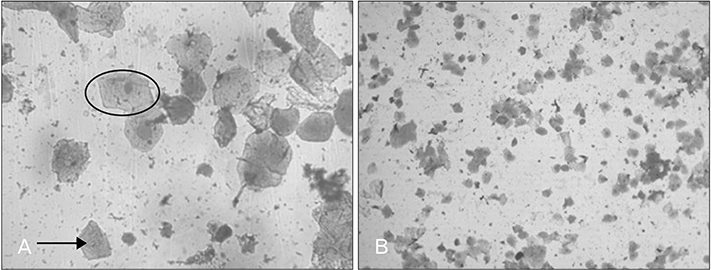
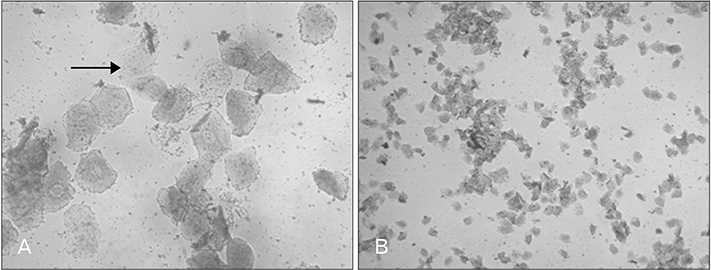
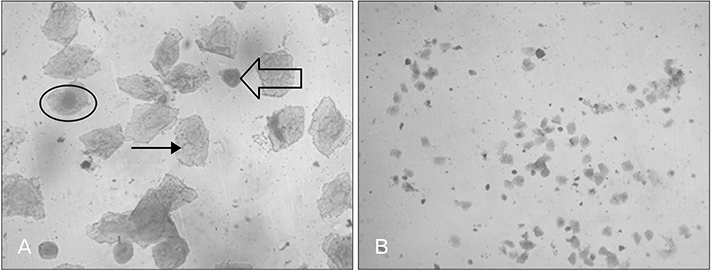
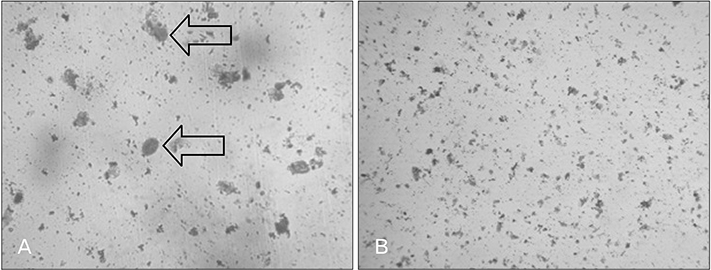
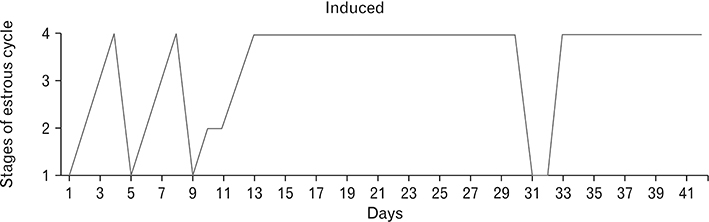
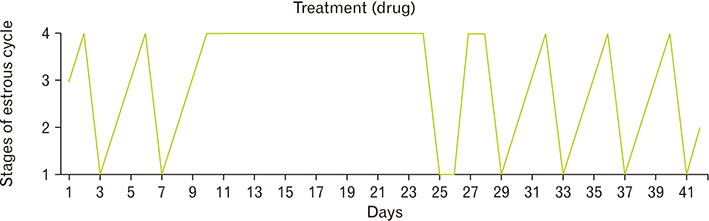
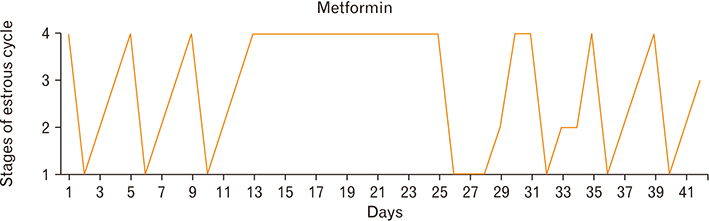
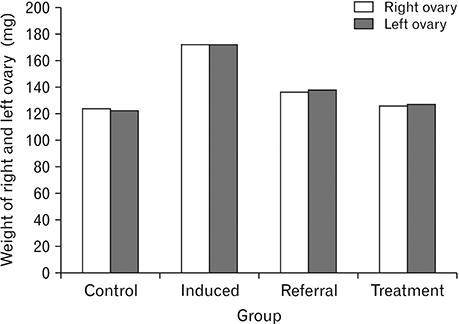
- Menstrual cycle is controlled by luteinizing hormone (LH) and follicle-stimulating hormone of anterior pituitary and regulated by gonadotropin-releasing hormone of hypothalamus. Any disturbance to this regulatory mechanism alters the pulsatile release of these hormones, especially LH; leads to polycystic ovarian syndrome (PCOS). Changes in vaginal cytology are used to interpret the changes in hormonal levels and modifications in estrous cycle. The aim of this study is to compare the pattern of vaginal cytology and body mass among PCOS rats which are treated with metformin and Cynodon dactylon. Twenty-four Wistar rats were selected and divided into four groups: control, induced, treatment, and referral. PCOS was induced in all groups except controls by giving letrozole through oral gavage for 21 days. After inducing PCOS, the referral and treatment group were treated for PCOS with metformin and C. dactylon respectively for next 21 days. Vaginal smear of all the groups were taken every day from day one and screened for estrous cycle. The body mass of the animals was measured on days 1, 21, and 42. Animals were sacrificed after 24 hours of the last dose and the reproductive organs were dissected out and weighed. Results of the study show the estrous cycle begins to revert after 1-week administration of C. dactylon; while the changes were slower in referral group. There was a rapid decrease in the body mass as well as reproductive organs among the treatment and referral group compared to that of induced and control. Finding of this study suggests that C. dactylon treats PCOS better than metformin.
MeSH Terms
-
Animals
Cynodon*
Estrous Cycle*
Female
Follicle Stimulating Hormone
Gonadotropin-Releasing Hormone
Hypothalamus
Luteinizing Hormone
Menstrual Cycle
Metformin
Polycystic Ovary Syndrome*
Rats*
Rats, Wistar
Referral and Consultation
Vaginal Smears
Follicle Stimulating Hormone
Gonadotropin-Releasing Hormone
Luteinizing Hormone
Metformin
Figure
Reference
-
1. Reame N, Sauder SE, Kelch RP, Marshall JC. Pulsatile gonadotropin secretion during the human menstrual cycle: evidence for altered frequency of gonadotropin-releasing hormone secretion. J Clin Endocrinol Metab. 1984; 59:328–337.2. Wildt L, Hausler A, Marshall G, Hutchison JS, Plant TM, Belchetz PE, Knobil E. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology. 1981; 109:376–385.3. Hayes FJ, Hall JE, Boepple PA, Crowley WF Jr. Clinical review 96: differential control of gonadotropin secretion in the human: endocrine role of inhibin. J Clin Endocrinol Metab. 1998; 83:1835–1841.4. Roe AH, Dokras A. The diagnosis of polycystic ovary syndrome in adolescents. Rev Obstet Gynecol. 2011; 4:45–51.5. Azziz R. Controversy in clinical endocrinology: diagnosis of polycystic ovarian syndrome. The Rotterdam criteria are premature. J Clin Endocrinol Metab. 2006; 91:781–785.6. Hirsutism and polycystic ovary syndrome (PCOS) [Internet]. Burmingham, AL: American Society for Reproductive Medicine;2016. cited 2018 Sep 10. Available from: https://www.reproductivefacts.org/globalassets/rf/news-and-publications/bookletsfact-sheets/english-fact-sheets-and-info-booklets/booklet_hirsutism_and_pcos.pdf.7. Padmanabhan V, Veiga-Lopez A. Animal models of the polycystic ovary syndrome phenotype. Steroids. 2013; 78:734–740.8. Maliqueo M, Benrick A, Stener-Victorin E. Rodent models of polycystic ovary syndrome: phenotypic presentation, pathophysiology, and the effects of different interventions. Semin Reprod Med. 2014; 32:183–193.9. Byers SL, Wiles MV, Dunn SL, Taft RA. Mouse estrous cycle identification tool and images. PLoS One. 2012; 7:e35538.10. Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007; 80:84–97.11. Rojas-Sandoval J, Acevedo-Rodriguez P. Cynodon dactylon (Bermuda grass) [Internet]. Wallingford: CAB International;2018. cited 2018 Sep 10. Available from: https://www.cabi.org/isc/datasheet/17463.12. Kaup SR, Arunkumar N, Bernhardt LK, Vasavi RG, Shetty SS, Pai SR, Arunkumar B. Antihyperlipedemic activity of Cynodon dactylon extract in high-cholesterol diet fed Wistar rats. Genomic Med Biomark Health Sci. 2011; 3:98–102.13. Kanimozhi DM, Bai RR. In vitro anticancer activity of ethanolic extract of Cynodon dactylon against HT-29 cell line. Int J Curr Sci. 2013; 5:74–81.14. Karthik D, Ravikumar S. A study on the protective effect of Cynodon dactylon leaves extract in diabetic rats. Biomed Environ Sci. 2011; 24:190–199.15. Ramesh H. Preclinical evaluation of protective effect of Cynodon dactylon pers on experimentally induced gastric mucosal damage. Res Rev J Med Health Sci. 2013; 2:89–93.16. Devi HC, Moirangthem RS, Vikram SS, Devi KS, Devi KP, Rita S. Hepatoprotective activity of aqueous extract of Cynodon dactylon on paracetamol induced hepatotoxic albino rats. Sch J Appl Med Sci. 2017; 5:199–204.17. Pandey K, Singh CS, Prasad RK, Singh AK, Mishra MK. Studies of anti-microbial activity using leaf extract of Cynodon dactylon. Pharm Lett. 2016; 8:325–330.18. Sharma D. Study of antimicrobial activity of Cynodon dactylon. Res Rev J Microbiol Biotechnol. 2016; 26–31.19. Pashaie B, Hobbenaghi R, Malekinejad H. Anti-atherosclerotic effect of Cynodon dactylon extract on experimentally induced hypercholesterolemia in rats. Vet Res Forum. 2017; 8:185–193.20. Part 5: Preparation, reading and reporting of vaginal smears [Internet]. Paris: Organisation for Economic Cooperation and Development;2018. cited 2018 Aug 10. Available from: http://www.oecd.org/chemicalsafety/testing/40581357.pdf.21. Reddy PS, Begum N, Mutha S, Bakshi V. Beneficial effect of Curcumin in Letrozole induced polycystic ovary syndrome. Asian Pac J Reprod. 2016; 5:116–122.22. Vani , Gopalan DH, Manikandan S, Vijayakumar V. Letrozole and fructose-induced polycystic ovaries in the rat: a novel model exhibiting both ovarian and metabolic characteristics for polycystic ovary syndrome in rat. Int J Pharm Sci Res. 2018; 9:2238–2243.23. Jahan S, Abid A, Khalid S, Afsar T, Qurat-Ul-Ain , Shaheen G, Almajwal A, Razak S. Therapeutic potentials of quercetin in management of polycystic ovarian syndrome using letrozole induced rat model: a histological and a biochemical study. J Ovarian Res. 2018; 11:26.24. Karateke A, Dokuyucu R, Dogan H, Ozgur T, Tas ZA, Tutuk O, Agturk G, Tumer C. Investigation of therapeutic effects of erdosteine on polycystic ovary syndrome in a rat model. Med Princ Pract. 2018; 27:515–522.25. Hong Y, Yin Y, Tan Y, Hong K, Jiang F, Wang Y. Effect of quercetin on biochemical parameters in letrozole-induced polycystic ovary syndrome in rats. Trop J Pharm Res. 2018; 17:1783–1788.26. Basheer M, Rai S, Ghosh H, Ahmad Hajam Y. Protective role of seed extract of Tephrosia purpurea in letrozole induced polycystic ovary syndrome in Wistar rats. J Biol Sci. 2018; 18:458–467.27. Abdollahifar MA, Azad N, Sajadi E, Shams Mofarahe Z, Zare F, Moradi A, Rezaee F, Gholamin M, Abdi S. Vitamin C restores ovarian follicular reservation in a mouse model of aging. Anat Cell Biol. 2019; 52:196–203.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Beneficial effects of Teucrium polium hydroalcoholic extract on letrozole-induced polycystic ovary syndrome in rat model
- Histologic comparison of polycystic ovary syndrome induced by estradiol valerate and letrozole
- The Efficacy of Letrozole in Women with a Poor Endometrial Response to Clomiphene Citrate
- Menstruation and Sleep
- Regulation of Estrogen Receptor mRNA in Rat Anterior Pituitary Gland