Anat Cell Biol.
2019 Dec;52(4):426-435. 10.5115/acb.19.111.
Topographical variations of the incisive canal and nasopalatine duct in human fetuses
- Affiliations
-
- 1Department of Anatomy, Jeonbuk National University Medical School, Jeonju, Korea. 407kk@hanmail.net
- 2Department of Maxillofacial Anatomy, Graduate School of Tokyo Medical and Dental University, Tokyo, Japan.
- 3Department of Anatomy, Akita University School of Medicine, Akita, Japan.
- 4Division of Internal Medicine, Jikoukai Clinic for Home Visit, Sapporo, Japan.
- 5Department of Anatomy and Human Embryology, Faculty of Medicine, Complutense University, Madrid, Spain.
- KMID: 2466696
- DOI: http://doi.org/10.5115/acb.19.111
Abstract
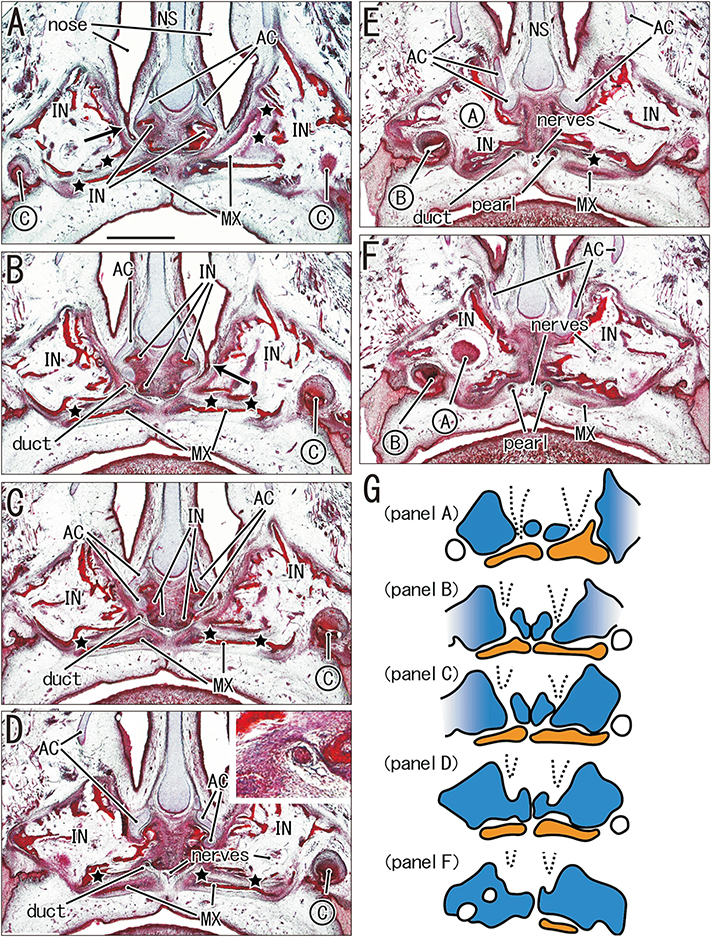
- The incisive canal for nerves and vessels is generally thought to run along a suture between the incisive bone (IN) and maxilla. In contrast, there was a report saying the canal passes through the IN or primary palate in human fetuses. Examination of sagittal and frontal sections from 69 fetuses (31 of gestational age [GA] 9-15 weeks and 38 of GA 26-34 weeks) showed that the canal often penetrated the IN at the nasal half of its course and that, in other fetuses, the canal penetrated the IN along its entire course, irrespective of involvement of the nasopalatine duct. Canals developing in and corresponding to parts of the suture resulted in partial enlargement of the thin and tight sutures, which contained loose tissue, vessels, nerves and even a duct. Small processes of the IN were identified as upper irregular parts continuous with inferior main masses of bone in frontal sections but as bone fragments in sagittal sections. In some sections, a thin layer of the maxilla along the canal covered the medial or inferior aspect of the IN. Therefore, the incisive canal with or without duct exhibited a spectrum of variations in topographical relation to the IN-maxillary border. Because the primitive oronasal communication passes through the suture, the nasopalatine duct may have originated from the secondary developed elongation of the nasal epithelium at midterm. A large incisive fossa along the midline on the oral surface of the palate might make a macroscopic finding of variants difficult even in adults.
Figure
Cited by 1 articles
-
Development and growth of the temporal fascia: a histological study using human fetuses
Kei Kitamura, Satoshi Ishizuka, Ji Hyun Kim, Hitoshi Yamamoto, Gen Murakami, Jose Francisco Rodríguez-Vázquez, Shin-ichi Abe
Anat Cell Biol. 2024;57(2):288-293. doi: 10.5115/acb.23.298.
Reference
-
1. Williams PL. Gray's anatomy. 38th ed. London: Churchill Livingstone;1995. p. 602.2. Njio BJ, Kjaer I. The development and morphology of the incisive fissure and the transverse palatine suture in the human fetal palate. J Craniofac Genet Dev Biol. 1993; 13:24–34.3. Vacher C, Sakka M, Dauge MC. Incisive suture (fissure) in the human fetus: radiologic and histologic study. Cleft Palate Craniofac J. 2001; 38:330–336.4. Barteczko K, Jacob M. A re-evaluation of the premaxillary bone in humans. Anat Embryol (Berl). 2004; 207:417–437.5. Melsen B, Melsen F. The postnatal development of the palatomaxillary region studied on human autopsy material. Am J Orthod. 1982; 82:329–342.6. Silau AM, Njio B, Solow B, Kjaer I. Prenatal sagittal growth of the osseous components of the human palate. J Craniofac Genet Dev Biol. 1994; 14:252–256.7. Rojvachiranonda N, Tansatit T, Siriwan P, Mahatumarat C. Normal palatal sutures in newborns and fetuses: a critical fact for successful palatal distraction. J Craniofac Surg. 2003; 14:457–461.8. Radlanski RJ, Emmerich S, Renz H. Prenatal morphogenesis of the human incisive canal. Anat Embryol (Berl). 2004; 208:265–271.9. Kim JH, Oka K, Jin ZW, Murakami G, Rodríguez-Vázquez JF, Ahn SW, Hwang HP. Fetal development of the incisive canal, especially of the delayed closure due to the nasopalatine duct: a study using serial sections of human fetuses. Anat Rec (Hoboken). 2017; 300:1093–1103.10. Wood PJ, Kraus BS. Prenatal development of the human palate. Some histological observations. Arch Oral Bio. 1962; 7:137–150.11. Kim JH, Jin ZW, Shibata S, Yang JD, Murakami G, Rodríguez-Vázquez JF, Cho BH. Fetal development of human oral epithelial pearls with special reference to their stage-dependent changes in distribution. Cleft Palate Craniofac J. 2017; 54:295–303.12. Kitamura H. Evidence for cleft palate as a postfusion phenomenon. Cleft Palate Craniofac J. 1991; 28:195–210.13. Arnold WH, Rezwani T, Baric I. Location and distribution of epithelial pearls and tooth buds in human fetuses with cleft lip and palate. Cleft Palate Craniofac J. 1998; 35:359–365.14. Kjaer I. Prenatal skeletal maturation of the human maxilla. J Craniofac Genet Dev Biol. 1989; 9:257–264.15. Kumoi T, Nishimura Y, Shiota K. The embryologic development of the human anterior nasal aperture. Acta Otolaryngol. 1993; 113:93–97.16. Sandikcioglu M, Mølsted K, Kjaer I. The prenatal development of the human nasal and vomeral bones. J Craniofac Genet Dev Biol. 1994; 14:124–134.17. Carstens MH. Development of the facial midline. J Craniofac Surg. 2002; 13:129–187.18. Hansen L, Nolting D, Holm G, Hansen BF, Kjaer I. Abnormal vomer development in human fetuses with isolated cleft palate. Cleft Palate Craniofac J. 2004; 41:470–473.19. Neves FS, Crusoé-Souza M, Franco LC, Caria PH, Bonfim-Almeida P, Crusoé-Rebello I. Canalis sinuosus: a rare anatomical variation. Surg Radiol Anat. 2012; 34:563–566.20. Neves FS, Oliveira LK, Ramos Mariz AC, Crusoé-Rebello I, de Oliveira-Santos C. Rare anatomical variation related to the nasopalatine canal. Surg Radiol Anat. 2013; 35:853–855.21. von Arx T, Lozanoff S, Sendi P, Bornstein MM. Assessment of bone channels other than the nasopalatine canal in the anterior maxilla using limited cone beam computed tomography. Surg Radiol Anat. 2013; 35:783–790.22. Etoz M, Sisman Y. Evaluation of the nasopalatine canal and variations with cone-beam computed tomography. Surg Radiol Anat. 2014; 36:805–812.23. Sekerci AE, Buyuk SK, Cantekin K. Cone-beam computed tomographic analysis of the morphological characterization of the nasopalatine canal in a pediatric population. Surg Radiol Anat. 2014; 36:925–932.24. Fernández-Alonso A, Suárez-Quintanilla JA, Rapado-González O, Suárez-Cunqueiro MM. Morphometric differences of nasopalatine canal based on 3D classifications: descriptive analysis on CBCT. Surg Radiol Anat. 2015; 37:825–833.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Clinical Experience of Nasopalatine Duct Cyst with Bony Defect
- A Case of Huge Nasopalatine Duct Cyst With Infection
- Anatomy and morphology of the nasopalatine canal using cone-beam computed tomography
- Two Cases of Marsupialization of Nasopalatine Duct Cyst
- Anatomic study of the incisive canal in relation to midpalatal placement of mini-implant