Infect Chemother.
2016 Sep;48(3):219-224. 10.3947/ic.2016.48.3.219.
Efficacy and Safety of Elvitegravir/Cobicistat/Emtricitabine/Tenofovir Disoproxil Fumarate in Asian Subjects with Human Immunodeficiency Virus 1 Infection: A Sub-Analysis of Phase 3 Clinical Trials
- Affiliations
-
- 1Department of Internal Medicine, Severance Hospital, Yonsei University, College of Medicine, Seoul, Korea. seran@yuhs.ac
- 2Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand.
- 3Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand.
- 4Brigham and Women’s Hospital. Boston, MA, USA.
- 5Orlando Immunology Center, Orlando, FL, USA.
- 6Highland General Hospital, Oakland, CA, USA.
- 7Chelsea and Westminster Hospital, London, UK.
- 8Gilead Sciences, Inc Foster City, CA, USA.
- 9Gilead Sciences Korea Ltd, Seoul, Korea.
- KMID: 2466435
- DOI: http://doi.org/10.3947/ic.2016.48.3.219
Abstract
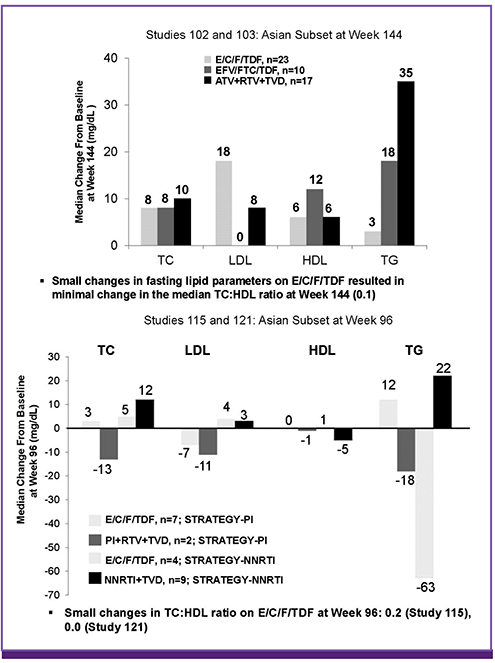
- The efficacy and safety of a single tablet regimen (STR) of elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate (E/C/F/TDF) were analyzed in Phase 3 clinical trials in antiretroviral therapy (ART)-naïve and ART-experienced Asian subjects infected with human immunodeficiency virus (HIV)-1. Studies GS-US-236-102 and GS-US-236-103 were randomized, double-blind, placebo-controlled, 144-week studies conducted in ART-naïve subjects, comparing E/C/F/TDF versus efavirenz (EFV)/F/TDF or ritonavir-boosted atazanavir (ATV+RTV) plus emtricitabine/tenofovir DF (F/TDF), respectively. Studies GS-US-236-115 and GS-US-236-121 were randomized, open-label, 96-week long conducted in ART-experienced subjects, who switched to E/C/F/TDF from ritonavir-boosted protease inhibitors (PI+RTV)+F/TDF, or non-nucleoside reverse transcriptase inhibitors (NNRTI)+F/TDF regimens. The E/C/F/TDF appeared to have sustained efficacy and safety and was well tolerated in the small number of ART-naïve and ART-experienced Asian subjects.
Keyword
MeSH Terms
Figure
Reference
-
1. Department for Health and Human Services (DHHS). Guidelines for the Use of Antiretroviral Agents in HIV-1 Infected Adults and Adolescents. Accessed 28 January 2016. Available at: https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-treatment-guidelines/0.2. European AIDS Clinical Society (EACS). EACS Guidelines Version 8.0. Accessed 10 January 2016. Available at: http://www.eacsociety.org/guidelines/eacs-guidelines/eacs-guidelines.html.3. Sax PE, DeJesus E, Mills A, Zolopa A, Cohen C, Wohl D, Gallant JE, Liu HC, Zhong L, Yale K, White K, Kearney BP, Szwarcberg J, Quirk E, Cheng AK. GS-US-236-0102 study team. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet. 2012; 379:2439–2448.
Article4. DeJesus E, Rockstroh JK, Henry K, Molina JM, Gathe J, Ramanathan S, Wei X, Yale K, Szwarcberg J, White K, Cheng AK, Kearney BP. GS-236-0103 Study Team. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate versus ritonavir-boosted atazanavir plus co-formulated emtricitabine and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet. 2012; 379:2429–2438.
Article5. Zolopa A, Sax PE, DeJesus E, Mills A, Cohen C, Wohl D, Gallant JE, Liu HC, Plummer A, White KL, Cheng AK, Rhee MS, Szwarcberg J. GS-US-236-0102 Study Team. A randomized double-blind comparison of coformulated elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate versus efavirenz/emtricitabine/tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: analysis of week 96 results. J Acquir Immune Defic Syndr. 2013; 63:96–100.
Article6. Rockstroh JK, DeJesus E, Henry K, Molina JM, Gathe J, Ramanathan S, Wei X, Plummer A, Abram M, Cheng AK, Fordyce MW, Szwarcberg J. GS-236-0103 Study Team. A randomized, double-blind comparison of co-formulated elvitegravir/cobicistat/emtricitabine/tenofovir versus ritonavir-boosted atazanavir plus co-formulated emtricitabine and tenofovir DF for initial treatment of HIV-1 infection: analysis of week 96 results. J Acquir Immune Defic Syndr. 2013; 62:483–486.
Article7. Rockstroh J. A randomized, double-blind comparison of coformulated elvitegravir/cobicistat/emtricitabine/tenofovir DF vs ritonavir-boosted atazanavir plus coformulated emtricitabine and tenofovir DF for initial treatment of HIV-1 infection: analysis of week 96 results. [erratum]. J Acquir Immune Defic Syndr. 2013; 63:e171.8. Wohl DA, Cohen C, Gallant JE, Mills A, Sax PE, Dejesus E, Zolopa A, Liu HC, Plummer A, White KL, Cheng AK, Rhee MS, Szwarcberg J. GS-US-236-0102 Study Team. A randomized, double-blind comparison of single-tablet regimen elvitegravir/cobicistat/emtricitabine/tenofovir DF versus single-tablet regimen efavirenz/emtricitabine/renofovir DF for initial treatment of HIV-1 infection: analysis of week 144 results. J Acquir Immune Defic Syndr. 2014; 65:e118–20.9. Clumeck N, Molina JM, Henry K, Gathe J, Rockstroh JK, DeJesus E, Wei X, White K, Fordyce MW, Rhee MS, Szwarcberg J. GS-236-0103 Study Team. A randomized, double-blind comparison of single-tablet regimenelvitegravir/cobicistat/emtricitabine/tenofovir DF vs ritonavir-boosted atazanavir plusemtricitabine/tenofovir DF for initial treatment of HIV-1 infection: analysis of week144 results. J Acquir Immune Defic Syndr. 2014; 65:e121–4.10. Arribas JR, Pialoux G, Gathe J, Di Perri G, Reynes J, Tebas P, Nguyen T, Ebrahimi R, White K, Piontkowsky D. Simplification to coformulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus continuation of ritonavir-boosted protease inhibitor with emtricitabine and tenofovir in adults with virologically suppressed HIV (STRATEGY-PI): 48 week results of a randomised, open-label, phase 3b, non-inferiority trial. Lancet Infect Dis. 2014; 14:581–589.
Article11. Pozniak A, Markowitz M, Mills A, Stellbrink HJ, Antela A, Domingo P, Girard PM, Henry K, Nguyen T, Piontkowsky D, Garner W, White K, Guyer B. Switching to coformulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus continuation of non-nucleoside reverse transcriptase inhibitor with emtricitabine and tenofovir in virologically suppressed adults with HIV (STRATEGY-NNRTI): 48 week results of a randomised, open-label, phase 3b non-inferiority trial. Lancet Infect Dis. 2014; 14:590–599.
Article12. In : Arribas J, DeJesus E, Van Lunzen J, Zurawski C, Doroana M, Towner W, Lazzarin A, Nelson M, Benn P, Chu H, McColl D, Swamy R, Nguyen T, editors. Simplification to the STRIBILD single tablet regimen from PI + RTV + FTC/TDF multi-pill regimens maintains durable HIV suppression: week 96 results of STRATEGY-PI (Study 115) [Poster P1]. 21st Annual Conference of the British HIV Association; 2015 April 21-24; Brighton, UK.13. In : Pozniak A, Flamm J, Antinori A, Bloch M, Ward D, Berenguer J, Cote P, Smith A, Andreatta K, Garner W, Szwarcberg J, Piontkowsky D, editors. Switch to STRIBILD from NNRTI plus FTC/TDF regimens maintains HIV suppression and is well-tolerated: week 96 results of STRATEGY-NNRTI (Study 121) [Poster P5]. 21st Annual Conference of the British HIV Association; 2015 April 21-24; Brighton, UK.14. Gathe J, Arribas JR, Van Lunzen J, Garner W, Speck RM, Bender R, Shreay S, Nguyen T. Patient-reported symptoms over 48 weeks in a randomized, open-label, phase 3b non-inferiority rrial of adults with HIV switching to coformulated elvitegravir, cobicistat, emtricitabine, and tenofovir DF versus continuation of ritonavir-boosted protease inhibitor with emtricitabine and tenofovir DF. Patient. 2015; 8:445–454.
Article15. Mills A, Garner W, Pozniak A, Berenguer J, Speck RM, Bender R, Nguyen T. Patient-reported symptoms over 48 weeks in a randomized, open-label, phase IIIb non-inferiority trial of adults with HIV switching to co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir DF versus continuation of non-nucleoside reverse transcriptase inhibitor with emtricitabine and tenofovir DF. Patient. 2015; 8:359–371.
Article16. In : Choi JY, Sungkanuparaph S, Anekthananon T, Sax P, DeJesus E, Edelstein H, Nelson M, DeMorin J, Liu H, Swamy R, Bahn J, Ng C, Piontkowsky D, editors. Efficacy and safety of elvitegravir/cobicistat/emtricitabine/tenofovir DF in HIV-1 infected, Asian subjects: a sub-analysis of phase 3 clinical trials [Presentation OB-6]. Interscience Conference on Infection and Chemotherapy (ICIC); 2015 November 5-7; Seoul, Korea.17. Cohen C, Elion R, Ruane P, Shamblaw D, DeJesus E, Rashbaum B, Chuck SL, Yale K, Liu HC, Warren DR, Ramanathan S, Kearney BP. Randomized, phase 2 evaluation of two single-tablet regimens elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate versus efavirenz/emtricitabine/tenofovir disoproxil fumarate for the initial treatment of HIV infection. AIDS. 2011; 25:F7–12.
Article18. German P, Lui C, Warren D, Hepner-Harris M, Andrews J, Kearney BP, Mathias A. Effect of cobicistat on glomerular filtration rate (GFR) in subjects with normal and impaired renal function [Poster H2-804]. In : 51st Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); 2011 September 17-20; Chicago, IL, USA.19. Elion R, Cohen C, Gathe J, Shalit P, Hawkins T, Liu HC, Mathias AA, Chuck SL, Kearney BP, Warren DR. GS-US-216-0105 Study Team. Phase 2 study of cobicistat versus ritonavir each with once-daily atazanavir and fixed-dose emtricitabine/tenofovir df in the initial treatment of HIV infection. AIDS. 2011; 25:1881–1886.
Article20. German P, Liu HC, Szwarcberg J, Hepner M, Andrews J, Kearney BP, Mathias A. Effect of cobicistat on glomerular filtration rate in subjects with normal and impaired renal function. J Acquir Immune Defic Syndr. 2012; 61:32–40.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recovery of Tenofovir-induced Nephrotoxicity following Switch from Tenofovir Disoproxil Fumarate to Tenofovir Alafenamide in Human Immunodeficiency Virus-Positive Patients
- Comparative pharmacokinetics between tenofovir disoproxil phosphate and tenofovir disoproxil fumarate in healthy subjects
- Angle's Class II Division 2 Malocclusion Treated by Bioprogressive Mechanism: Report of a Case
- Clinical and radiobiological consideration of cyclical hypofractionated radiation therapy also known as QUAD Shot for neglected skin cancer disfiguring the face of a non-compliant patient who was refusing surgery and protracted radiation therapy: case report
- Similar Durability of Two Single Tablet Regimens, Dolutegravir/Abacavir/Lamivudine and Elvitegravir/Cobicistat/Tenofovir/Emtricitabine: Single Center Experience