Ann Lab Med.
2020 May;40(3):216-223. 10.3343/alm.2020.40.3.216.
Development and Validation of a Novel Warfarin Dosing Algorithm for Korean Patients With VKORC1 1173C
- Affiliations
-
- 1Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. suddenbz@skku.edu
- 2Department of Laboratory Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Laboratory Medicine, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea.
- 4Department of Laboratory Medicine, Green Cross Laboratories, Yongin, Korea.
- 5Statistics and Data Center, Samsung Medical Center, Seoul, Korea.
- 6Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 7Department of Neurology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 8Department of Laboratory Medicine, Konyang University Hospital, Konyang University School of Medicine, Daejeon, Korea. hjchomd@kyuh.ac.kr
- 9Department of Clinical Pharmacology & Therapeutics, Samsung Medical Center, Seoul, Korea.
- 10Department of Health Sciences and Technology, SAIHST, Sungkyunkwan University, Seoul, Korea.
- KMID: 2466014
- DOI: http://doi.org/10.3343/alm.2020.40.3.216
Abstract
- BACKGROUND
Differences in the performance of suggested warfarin dosing algorithms among different ethnicities and genotypes have been reported; this necessitates the development of an algorithm with enhanced performance for specific population groups. Previous warfarin dosing algorithms underestimated warfarin doses in VKORC1 1173C carriers. We aimed to develop and validate a new warfarin dosing algorithm for Korean patients with VKORC1 1173C.
METHODS
A total of 109 patients carrying VKORC1 1173CT (N=105) or 1173CC (N=4) were included in this study. Multiple regression analysis was performed to deduce a new dosing algorithm. Following literature searches for genotype-guided warfarin dosing algorithms, 21 algorithms were selected and evaluated using the correlation coefficient (Ï) of actual dose and estimated dose, mean error, and root mean square error.
RESULTS
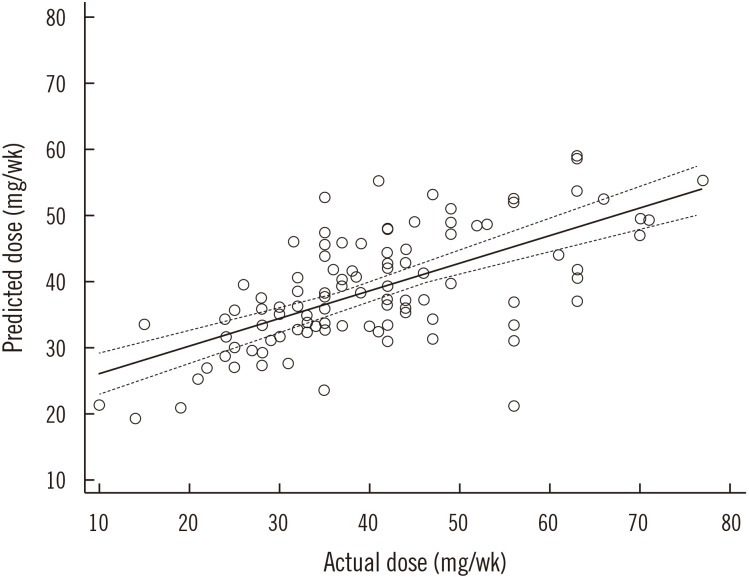
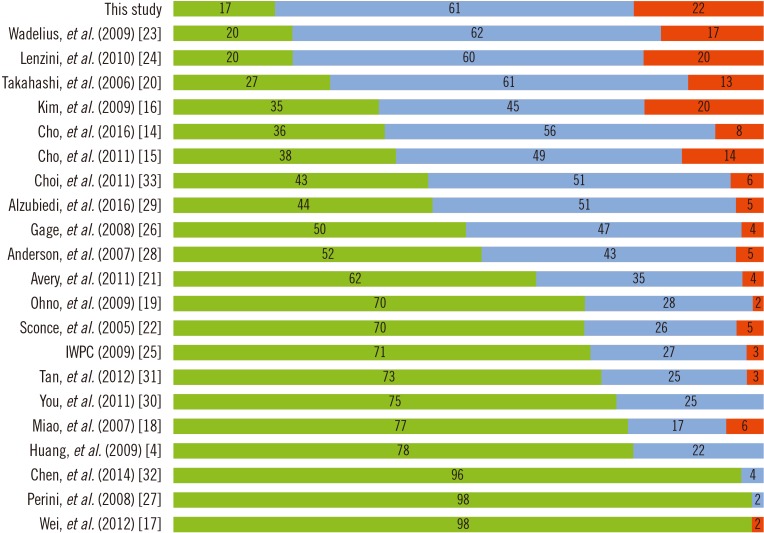
The developed algorithm is as follows: maintenance dose (mg/week)=exp [3.223−0.009×(age)+0.577×(body surface area [BSA])+0.178×(sex)−0.481×(CYP2C9 genotype)+0.227×(VKORC1 genotype)]. Integrated variables explained 44% of the variance in the maintenance dose. The predicted and actual doses showed moderate correlation (Ï=0.641) with the best performance with a mean error of −1.30 mg/week. The proportion of underestimated groups was 17%, which was lower than with the other algorithms.
CONCLUSIONS
This is the first study to develop and validate a warfarin dosing algorithm based on data from VKORC1 1173C carriers; it showed superior predictive performance compared with previously published algorithms.
Keyword
Figure
Reference
-
1. Shaw K, Amstutz U, Kim RB, Lesko LJ, Turgeon J, Michaud V, et al. Clinical practice recommendations on genetic testing of CYP2C9 and VKORC1 variants in warfarin therapy. Ther Drug Monit. 2015; 37:428–436. PMID: 26186657.2. Kim S, Yun YM, Chae HJ, Cho HJ, Ji M, Kim IS, et al. Clinical pharmacogenetic testing and application: laboratory medicine clinical practice guidelines. Ann Lab Med. 2017; 37:180–193. PMID: 28029011.3. Verhoef TI, Redekop WK, Daly AK, van Schie RM, de Boer A, Maitland-van der Zee AH. Pharmacogenetic-guided dosing of coumarin anticoagulants: algorithms for warfarin, acenocoumarol and phenprocoumon. Br J Clin Pharmacol. 2014; 77:626–641. PMID: 23919835.4. Huang SW, Chen HS, Wang XQ, Huang L, Xu DL, Hu XJ, et al. Validation of VKORC1 and CYP2C9 genotypes on interindividual warfarin maintenance dose: a prospective study in Chinese patients. Pharmacogenet Genomics. 2009; 19:226–234. PMID: 19177029.5. Peng Q, Huang S, Chen X, Yuan Y, Yu Y, Tao L, et al. Validation of warfarin pharmacogenetic algorithms in 586 Han Chinese patients. Pharmacogenomics. 2015; 16:1465–1474. PMID: 26255607.6. Yang M, Choi R, Kim JS, On YK, Bang OY, Cho HJ, et al. Evaluation of 16 genotype-guided warfarin dosing algorithms in 310 Korean patients receiving warfarin treatment: poor prediction performance in VKORC1 1173C carriers. Clin Ther. 2016; 38:2666–2674.e1. PMID: 27887741.7. Saffian SM, Duffull SB, Wright D. Warfarin dosing algorithms underpredict dose requirements in patients requiring ≥7 mg daily: a systematic review and meta-analysis. Clin Pharmacol Ther. 2017; 102:297–304. PMID: 28160278.8. Limdi NA, Brown TM, Yan Q, Thigpen JL, Shendre A, Liu N, et al. Race influences warfarin dose changes associated with genetic factors. Blood. 2015; 126:539–545. PMID: 26024874.9. Rieder MJ, Reiner AP, Gage BF, Nickerson DA, Eby CS, McLeod HL, et al. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med. 2005; 352:2285–2293. PMID: 15930419.10. Patillon B, Luisi P, Blanché H, Patin E, Cann HM, Génin E, et al. Positive selection in the chromosome 16 VKORC1 genomic region has contributed to the variability of anticoagulant response in humans. PLoS One. 2012; 7:e53049. PMID: 23285254.11. D'Andrea G, D'Ambrosio RL, Di Perna P, Chetta M, Santacroce R, Brancaccio V, et al. A polymorphism in the VKORC1 gene is associated with an interindividual variability in the dose-anticoagulant effect of warfarin. Blood. 2005; 105:645–649. PMID: 15358623.12. Cho HJ, Sohn KH, Park HM, Lee KH, Choi B, Kim S, et al. Factors affecting the interindividual variability of warfarin dose requirement in adult Korean patients. Pharmacogenomics. 2007; 8:329–337. PMID: 17391071.13. Simon R, Radmacher MD, Dobbin K, McShane LM. Pitfalls in the use of DNA microarray data for diagnostic and prognostic classification. J Natl Cancer Inst. 2003; 95:14–18. PMID: 12509396.14. Cho SM, Lee KY, Choi JR, Lee KA. Development and comparison of warfarin dosing algorithms in stroke patients. Yonsei Med J. 2016; 57:635–640. PMID: 26996562.15. Cho HJ, On YK, Bang OY, Kim JW, Huh W, Ko JW, et al. Development and comparison of a warfarin-dosing algorithm for Korean patients with atrial fibrillation. Clin Ther. 2011; 33:1371–1380. PMID: 21981797.16. Kim HS, Lee SS, Oh M, Jang YJ, Kim EY, Han IY, et al. Effect of CYP2C9 and VKORC1 genotypes on early-phase and steady-state warfarin dosing in Korean patients with mechanical heart valve replacement. Pharmacogenet Genomics. 2009; 19:103–112. PMID: 19077919.17. Wei M, Ye F, Xie D, Zhu Y, Zhu J, Tao Y, et al. A new algorithm to predict warfarin dose from polymorphisms of CYP4F2, CYP2C9 and VKORC1 and clinical variables: derivation in Han Chinese patients with non valvular atrial fibrillation. Thromb Haemost. 2012; 107:1083–1091. PMID: 22534826.18. Miao L, Yang J, Huang C, Shen Z. Contribution of age, body weight, and CYP2C9 and VKORC1 genotype to the anticoagulant response to warfarin: proposal for a new dosing regimen in Chinese patients. Eur J Clin Pharmacol. 2007; 63:1135–1141. PMID: 17899045.19. Ohno M, Yamamoto A, Ono A, Miura G, Funamoto M, Takemoto Y, et al. Influence of clinical and genetic factors on warfarin dose requirements among Japanese patients. Eur J Clin Pharmacol. 2009; 65:1097–1103. PMID: 19582440.20. Takahashi H, Wilkinson GR, Nutescu EA, Morita T, Ritchie MD, Scordo MG, et al. Different contributions of polymorphisms in VKORC1 and CYP2C9 to intra- and inter-population differences in maintenance dose of warfarin in Japanese, Caucasians and African-Americans. Pharmacogenet Genomics. 2006; 16:101–110. PMID: 16424822.21. Avery PJ, Jorgensen A, Hamberg AK, Wadelius M, Pirmohamed M, Kamali F, et al. A proposal for an individualized pharmacogenetics-based warfarin initiation dose regimen for patients commencing anticoagulation therapy. Clin Pharmacol Ther. 2011; 90:701–706. PMID: 22012312.22. Sconce EA, Khan TI, Wynne HA, Avery P, Monkhouse L, King BP, et al. The impact of CYP2C9 and VKORC1 genetic polymorphism and patient characteristics upon warfarin dose requirements: proposal for a new dosing regimen. Blood. 2005; 106:2329–2333. PMID: 15947090.23. Wadelius M, Chen LY, Lindh JD, Eriksson N, Ghori MJ, Bumpstead S, et al. The largest prospective warfarin-treated cohort supports genetic forecasting. Blood. 2009; 113:784–792. PMID: 18574025.24. Lenzini P, Wadelius M, Kimmel S, Anderson JL, Jorgensen AL, Pirmohamed M, et al. Integration of genetic, clinical, and INR data to refine warfarin dosing. Clin Pharmacol Ther. 2010; 87:572–578. PMID: 20375999.25. International Warfarin Pharmacogenetics Consortium. Klein TE, Altman RB, Eriksson N, Gage BF, Kimmel SE, et al. Estimation of the warfarin dose with clinical and pharmacogenetic data. N Engl J Med. 2009; 360:753–764. PMID: 19228618.26. Gage BF, Eby C, Johnson JA, Deych E, Rieder MJ, Ridker PM, et al. Use of pharmacogenetic and clinical factors to predict the therapeutic dose of warfarin. Clin Pharmacol Ther. 2008; 84:326–331. PMID: 18305455.27. Perini JA, Struchiner CJ, Silva-Assunção E, Santana IS, Rangel F, Ojopi EB, et al. Pharmacogenetics of warfarin: development of a dosing algorithm for Brazilian patients. Clin Pharmacol Ther. 2008; 84:722–728. PMID: 18754001.28. Anderson JL, Horne BD, Stevens SM, Grove AS, Barton S, Nicholas ZP, et al. Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation. Circulation. 2007; 116:2563–2570. PMID: 17989110.29. Alzubiedi S, Saleh MI. Pharmacogenetic-guided warfarin dosing algorithm in African-Americans. J Cardiovasc Pharmacol. 2016; 67:86–92. PMID: 26355760.30. You JH, Wong RS, Waye MM, Mu Y, Lim CK, Choi KC, et al. Warfarin dosing algorithm using clinical, demographic and pharmacogenetic data from Chinese patients. J Thromb Thrombolysis. 2011; 31:113–118. PMID: 20585834.31. Tan SL, Li Z, Song GB, Liu LM, Zhang W, Peng J, et al. Development and comparison of a new personalized warfarin stable dose prediction algorithm in Chinese patients undergoing heart valve replacement. Pharmazie. 2012; 67:930–937. PMID: 23210243.32. Chen J, Shao L, Gong L, Luo F, Wang J, Shi Y, et al. A pharmacogenetics-based warfarin maintenance dosing algorithm from Northern Chinese patients. PLoS One. 2014; 9:e105250. PMID: 25126975.33. Choi JR, Kim JO, Kang DR, Yoon SA, Shin JY, Zhang X, et al. Proposal of pharmacogenetics-based warfarin dosing algorithm in Korean patients. J Hum Genet. 2011; 56:290–295. PMID: 21326313.34. Mukaka MM. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 2012; 24:69–71. PMID: 23638278.35. Horne BD, Lenzini PA, Wadelius M, Jorgensen AL, Kimmel SE, Ridker PM, et al. Pharmacogenetic warfarin dose refinements remain significantly influenced by genetic factors after one week of therapy. Thromb Haemost. 2012; 107:232–240. PMID: 22186998.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of the Verigene Warfarin Metabolism Nucleic Acid Test Kit for the Rapid Detection of CYP2C9 and VKORC1 Polymorphisms
- Development and Comparison of Warfarin Dosing Algorithms in Stroke Patients
- Lack of Association of Clinical Factors (SAMe-TT2R2) with CYP2C9/VKORC1 Genotype and Anticoagulation Control Quality
- VKORC1 and CYP2C9 Genotype Variations in Relation to Warfarin Dosing in Korean Stroke Patients
- A child with Kawasaki disease and genetic warfarin sensitivity from CYP2C9 and VKORC1 gene variants