Ann Lab Med.
2020 May;40(3):201-208. 10.3343/alm.2020.40.3.201.
Clinical Application of Overlapping Confidence Intervals for Monitoring Changes in Serial Clinical Chemistry Test Results
- Affiliations
-
- 1Department of Laboratory Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea. u931018@yonsei.ac.kr
- 2Department of Medical Information, Yonsei University Wonju College of Medicine, Wonju, Korea.
- KMID: 2466012
- DOI: http://doi.org/10.3343/alm.2020.40.3.201
Abstract
- BACKGROUND
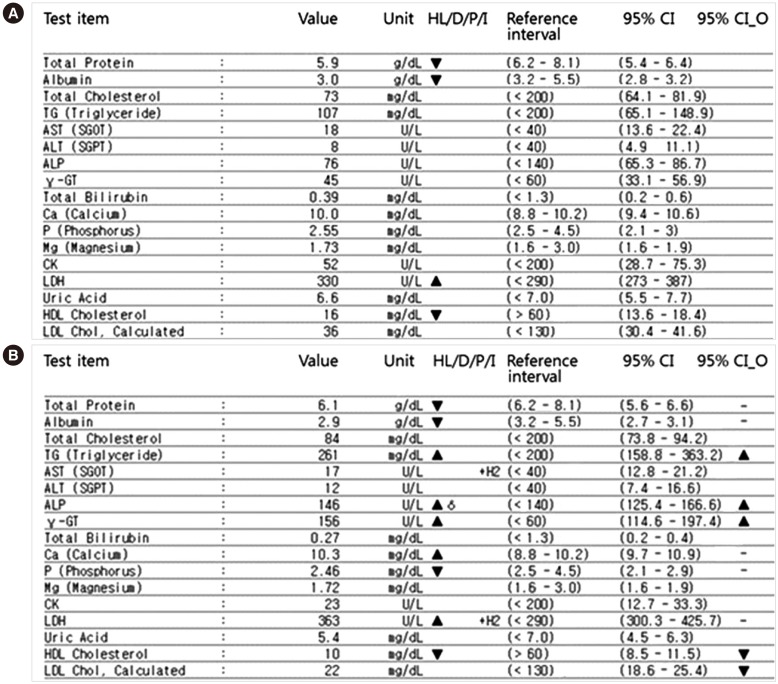
Interpretation of changes in serial laboratory results is necessary for both clinicians and laboratories; however, setting decision limits is not easy. Although the reference change value (RCV) has been widely used for auto-verification, it has limitations in clinical settings. We introduce the concept of overlapping confidence intervals (CIs) to determine whether the changes are statistically significant in clinical chemistry laboratory test results.
METHODS
In total, 1,202,096 paired results for 33 analytes routinely tested in our clinical chemistry laboratory were analyzed. The distributions of delta% absolute values and cut-off values for certain percentiles were calculated. The CIs for each analyte were set based on biological variation, and data were analyzed at various confidence levels. Additionally, we analyzed the data using RCVs and compared their clinical utility.
RESULTS
Most analytes had low indexes of individuality with large inter-individual variability. The 97.5th percentile cut-offs for each analyte were much larger than conventional RCVs. The percentages of results exceeding RCV(95%) and RCV(99%) corresponded to those with no overlap at the 83.4% and 93.2% confidence levels, respectively.
CONCLUSIONS
The use of overlapping CIs in serial clinical chemistry test results can overcome the limitations of existing RCVs and replace them, especially for analytes with large intra-individual variation.
Keyword
Figure
Reference
-
1. Nunes LA, Brenzikofer R, de Macedo DV. Reference change values of blood analytes from physically active subjects. Eur J Appl Physiol. 2010; 110:191–198. PMID: 20446091.2. Fraser CG. Making better use of differences in serial laboratory results. Ann Clin Biochem. 2012; 49:1–3. PMID: 22130633.3. Ricós C, Alvarez V, Cava F, García-Lario JV, Hernández A, Jiménez CV, et al. Current databases on biological variation: pros, cons and progress. Scand J Clin Lab Invest. 1999; 59:491–500. PMID: 10667686.4. Petersen PH, Fraser CG, Jørgensen L, Brandslund I, Stahl M, Gowans EM, et al. Combination of analytical quality specifications based on biological within- and between-subject variation. Ann Clin Biochem. 2002; 39:543–550. PMID: 12564835.5. Fraser CG, Hyltoft Peterson P, Larsen ML. Setting analytical goals for random analytical error in specific clinical monitoring situations. Clin Chem. 1990; 36:1625–1628. PMID: 2208703.6. Ricós C, Iglesias N, García-Lario JV, Simón M, Cava F, Hernández A, et al. Within-subject biological variation in disease: collated data and clinical consequences. Ann Clin Biochem. 2007; 44:343–352. PMID: 17594781.7. Bartlett WA, Braga F, Carobene A, Coşkun A, Prusa R, Fernandez-Calle P, et al. A checklist for critical appraisal of studies of biological variation. Clin Chem Lab Med. 2015; 53:879–885. PMID: 25996385.8. Widjaja A, Morris RJ, Levy JC, Frayn KN, Manley SE, Turner RC. Within- and between-subject variation in commonly measured anthropometric and biochemical variables. Clin Chem. 1999; 45:561–566. PMID: 10102917.9. Fraser CG. Data on biological variation: essential prerequisites for introducing new procedures? Clin Chem. 1994; 40:1671–1673. PMID: 8070075.10. Oosterhuis WP. Analytical performance specifications in clinical chemistry: the holy grail? J Lab Precis Med. 2017; 2:78.11. Lacher DA, Hughes JP, Carroll MD. Estimate of biological variation of laboratory analytes based on the third national health and nutrition examination survey. Clin Chem. 2005; 51:450–452. PMID: 15590751.12. Fernández-Grande E, Valera-Rodriguez C, Sáenz-Mateos L, Sastre-Gómez A, García-Chico P, Palomino-Muñoz TJ. Impact of reference change value (RCV) based autoverification on turnaround time and physician satisfaction. Biochem Med (Zagreb). 2017; 27:342–349. PMID: 28694725.13. Fraser CG. Reference change values. Clin Chem Lab Med. 2011; 50:807–812. PMID: 21958344.14. Ko DH, Park HI, Hyun J, Kim HS, Park MJ, Shin DH. Utility of reference change values for delta check limits. Am J Clin Pathol. 2017; 148:323–329. PMID: 28967949.15. Lee J, Kim SY, Kwon HJ, Lee HK, Kim Y, Kim Y. Usefulness of biological variation in the establishment of delta check limits. Clin Chim Acta. 2016; 463:18–21. PMID: 27524506.16. du Prel JB, Hommel G, Röhrig B, Blettner M. Confidence interval or p-value?: part 4 of a series on evaluation of scientific publications. Dtsch Arztebl Int. 2009; 106:335–339. PMID: 19547734.17. Hazra A. Using the confidence interval confidently. J Thorac Dis. 2017; 9:4125–4130. PMID: 29268424.18. European Federation of Clinical Chemistry and Laboratory Medicine. EFLM biological variation database. Updated on Aug 2019. https://biologicalvariation.eu.19. Westgard QC. Desirable biological variation database specifications. Updated on Jul 2019. https://www.westgard.com/biodatabase1.htm.20. Austin PC, Hux JE. A brief note on overlapping confidence intervals. J Vasc Surg. 2002; 36:194–195. PMID: 12096281.21. Knol MJ, Pestman WR, Grobbee DE. The (mis) use of overlap of confidence intervals to assess effect modification. Eur J Epidemiol. 2011; 26:253–254. PMID: 21424218.22. Jhang JS, Lifshitz MS. Post analysis: medical decision making. In : McPherson RA, Pincus MR, editors. Henry's clinical diagnosis and management by laboratory methods. 23rd ed. St. Louis, MO: Elsevier;2017. p. 73–83.23. Fraser CG. Inherent biological variation and reference values. Clin Chem Lab Med. 2004; 42:758–764. PMID: 15327011.24. Fraser CG, Petersen PH. Desirable standards for laboratory tests if they are to fulfill medical needs. Clin Chem. 1993; 39:1447–1453. PMID: 8330406.25. Park SH, Kim SY, Lee W, Chun S, Min WK. New decision criteria for selecting delta check methods based on the ratio of the delta difference to the width of the reference range can be generally applicable for each clinical chemistry test item. Ann Lab Med. 2012; 32:345–354. PMID: 22950070.26. Fuentes-Arderiu X. Variability of the biological variation. Scand J Clin Lab Invest. 2002; 62:561–563. PMID: 12512747.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Design and Laboratory Implementation of Web Application for Collaboratively Setting Reference Intervals
- Significant results: statistical or clinical?
- A Representative Value for 24-hr Ambulatory Blood pressure Monitoring
- Clinical chemistry values in elderly Korean people: single institutional study
- Development and Effects of Supplementary Material about Electronic Fetal Monitoring for Nursing Students