J Pathol Transl Med.
2019 Mar;53(2):129-135. 10.4132/jptm.2018.11.13.
Adrenal Cortical Neoplasm with Uncertain Malignant Potential Arising in the Heterotopic Adrenal Cortex in the Liver of a Patient with Beckwith-Wiedemann Syndrome
- Affiliations
-
- 1Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. ckim@amc.seoul.kr
- 2Department of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Department of Pediatrics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2465453
- DOI: http://doi.org/10.4132/jptm.2018.11.13
Abstract
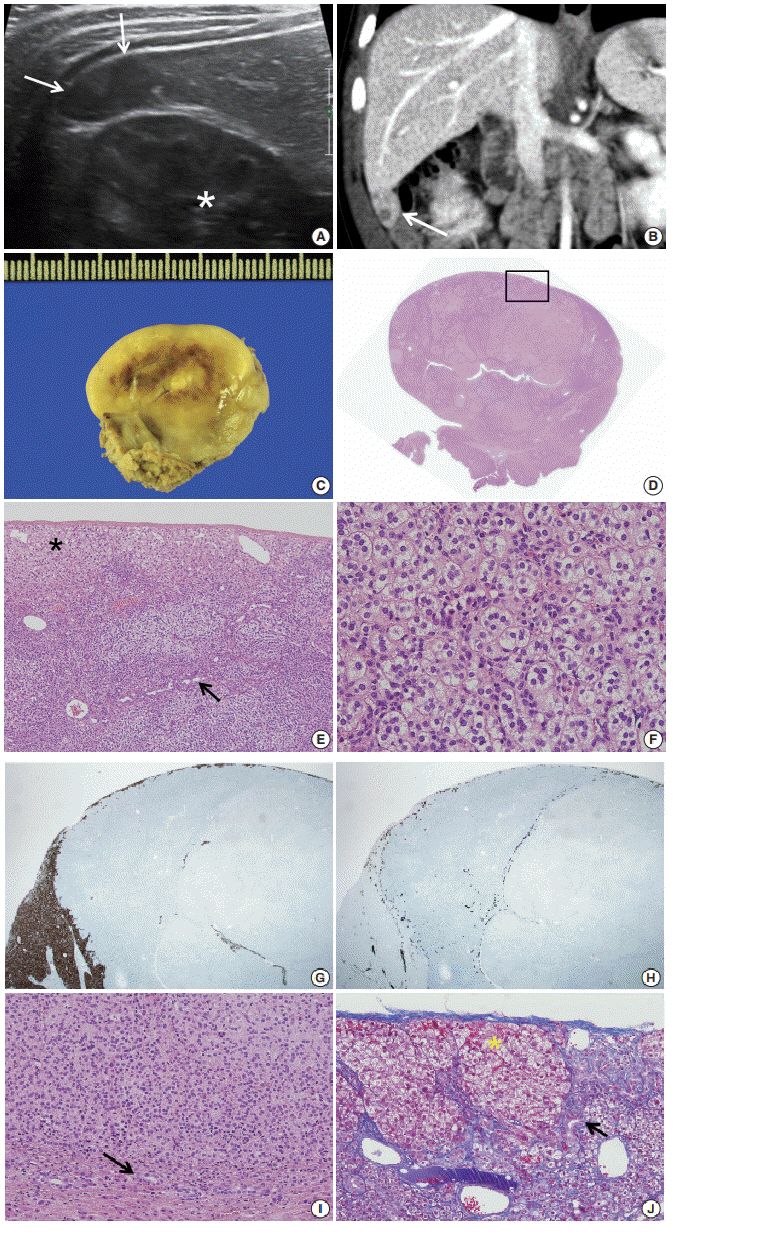
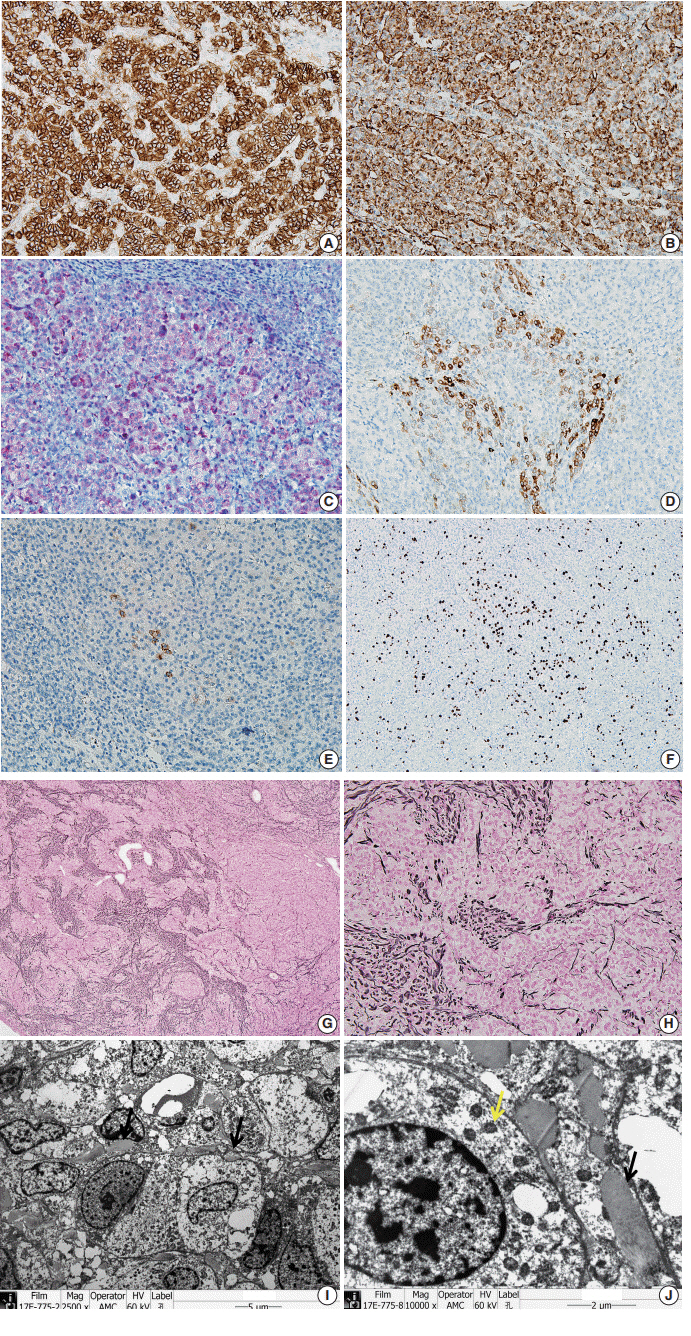
- Patients with Beckwith-Wiedemann syndrome (BWS) are predisposed to developing embryonal tumors, with hepatoblastoma being the most common type. Our patient showed hemihypertrophy, macroglossia, and paternal uniparental disomy in chromosome 11 and was diagnosed with BWS. When the patient was 9 months old, a 2.5×1.5 cm oval hypoechoic exophytic mass was detected in the inferior tip of his right liver. Preoperative imaging identified it as hepatoblastoma; however, histologic, immunohistochemistry, and electron microscopic findings were compatible with adrenal cortical neoplasm with uncertain malignant potential. The origin of the adrenal tissue seemed to be heterotopic. Here, we describe for the first time an adrenal cortical neoplasm with uncertain malignant potential arising in the heterotopic adrenal cortex located in the liver of a patient with BWS.
MeSH Terms
Figure
Reference
-
1. Shuman C, Beckwith JB, Weksberg R. Beckwith-Wiedemann syndrome. In : Adam MP, Ardinger HH, Pagon RA, editors. GeneReviews [Internet]. Seattle: University of Washington;c1993-2019 [cited 2018 Aug 2]. Available from: https://www.ncbi.nlm.nih.gov/pubmed/20301568.2. Pettenati MJ, Haines JL, Higgins RR, Wappner RS, Palmer CG, Weaver DD. Wiedemann-Beckwith syndrome: presentation of clinical and cytogenetic data on 22 new cases and review of the literature. Hum Genet. 1986; 74:143–54.
Article3. Sotelo-Avila C, Gooch WM 3rd. Neoplasms associated with the Beckwith-Wiedemann syndrome. Perspect Pediatr Pathol. 1976; 3:255–72.4. DeBaun MR, Tucker MA. Risk of cancer during the first four years of life in children from The Beckwith-Wiedemann Syndrome Registry. J Pediatr. 1998; 132(3 Pt 1):398–400.
Article5. Stocker JT, Dehner LP, Husain AN. Stocker and Dehner’s pediatric pathology. 3rd ed. Philadelphia: Lippincott Williams & Wilkins;2015.6. Graham LS. Celiac accessory adrenal glands. Cancer. 1953; 6:149–52.
Article7. Wilkins L, Ravitch MM. Adrenocortical tumor arising in the liver of a three year old boy with signs of virilism and Cushing’s syndrome: report of a case with cure after partial resection of the right lobe of the liver. Pediatrics. 1952; 9:671–81.8. Vestfrid MA. Ectopic adrenal cortex in neonatal liver. Histopathology. 1980; 4:669–72.
Article9. Honore LH, O'Hara KE. Combined adrenorenal fusion and adrenohepatic adhesion: a case report with review of the literature and discussion of pathogenesis. J Urol. 1976; 115:323–5.10. Honma K. Adreno-hepatic fusion: an autopsy study. Zentralbl Pathol. 1991; 137:117–22.11. Cardinalli IA, de Oliveira-Filho AG, Mastellaro MJ, Ribeiro RC, Aguiar SS. A unique case of synchronous functional adrenocortical adenoma and myelolipoma within the ectopic adrenal cortex in a child with Beckwith-Wiedemann syndrome. Pathol Res Pract. 2012; 208:189–94.
Article12. Giner J, Esteban I, Carceller F, Saceda J, Regojo RM. Spinal adrenal cortical adenoma associated with Beckwith-Wiedemann syndrome: case report and review of the literature. Childs Nerv Syst. 2017; 33:1009–13.
Article13. Wieneke JA, Thompson LD, Heffess CS. Adrenal cortical neoplasms in the pediatric population: a clinicopathologic and immunophenotypic analysis of 83 patients. Am J Surg Pathol. 2003; 27:867–81.