J Pathol Transl Med.
2019 Nov;53(6):361-368. 10.4132/jptm.2019.08.27.
Molecular and Clinicopathological Features of Gastrointestinal Stromal Tumors in Vietnamese Patients
- Affiliations
-
- 1Department of Pathology, University of Medicine and Pharmacy at Ho Chi Minh City, Ho Chi Minh City, Vietnam. ngoquocdat@ump.edu.vn
- 2Center for Molecular Biomedicine, University of Medicine and Pharmacy at Ho Chi Minh City, Ho Chi Minh City, Vietnam.
- KMID: 2465435
- DOI: http://doi.org/10.4132/jptm.2019.08.27
Abstract
- BACKGROUND
Gastrointestinal stromal tumors (GISTs) are the most frequent mesenchymal neoplasms of the gastrointestinal tract. Management of GIST patients is currently based on clinicopathological features and associated genetic changes. However, the detailed characteristics and molecular genetic features of GISTs have not yet been described in the Vietnamese population.
METHODS
We first identified 155 patients with primary GIST who underwent surgery with primary curative intent between 2011 and 2014 at University Medical Center at Ho Chi Minh City, Vietnam. We evaluated the clinicopathological features and immunohistochemical reactivity to p53 and Ki-67 in these patients. Additionally, KIT genotyping was performed in 100 cases.
RESULTS
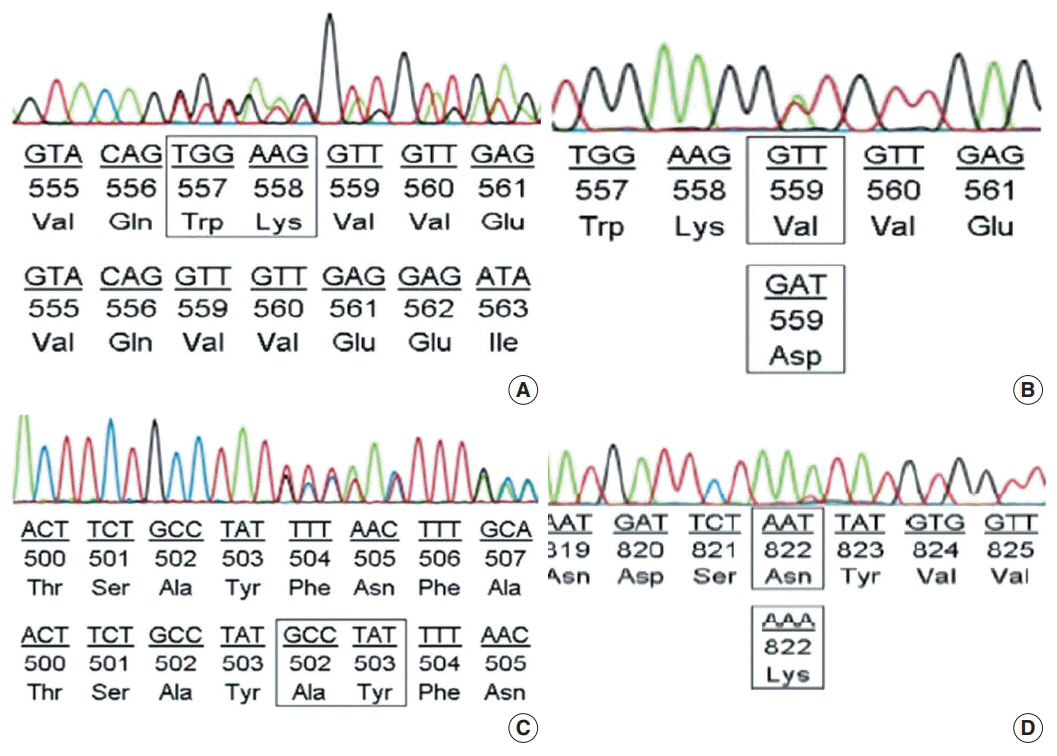
The largest proportion of GISTs was classified as high-risk (43.2%). Of the 155 GISTs, 52 (33.5%) were positive for Ki-67, and 58 (37.4%) were positive for p53. The expression of Ki-67 and p53 were correlated with mitotic rate, tumor size, risk assessment, and tumor stage. Out of 100 GIST cases, KIT mutation was found in 68%, of which 62 (91.2%) were found in exon 11, two (2.9%) in exon 9, and four (5.8%) in exon 17. No mutation in exon 13 was identified. Additionally, KIT mutations did not correlate with any clinicopathological features.
CONCLUSIONS
The expression of Ki-67 and p53 were associated with high-risk tumors. Mutations in exon 11 were the most commonly found, followed by exon 17 and exon 9. Additionally, KIT mutation status was not correlated with any recognized clinicopathological features.
Keyword
MeSH Terms
Figure
Reference
-
1. Miettinen M, Fletcher CD, Kindblom LG, Tsui WM. Mesenchymal tumours of the stomach. In : Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumours of the digestive system. 3rd. Lyon: IARC;2010. p. 74–9.2. Søreide K, Sandvik OM, Søreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): a systematic review of population-based cohort studies. Cancer Epidemiol. 2016; 40:39–46.
Article3. Mazur MT, Clark HB. Gastric stromal tumors: reappraisal of histogenesis. Am J Surg Pathol. 1983; 7:507–19.
Article4. Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-KIT in human gastrointestinal stromal tumors. Science. 1998; 279:577–80.5. Joensuu H. Gastrointestinal stromal tumor (GIST). Ann Oncol. 2006; 17 Suppl 10:x280–6.
Article6. Von Mehren M, Randall RL, Benjamin RS, et al. Gastrointestinal stromal tumors, version 2.2014. J Natl Compr Canc Netw. 2014; 12:853–62.
Article7. Miettinen M, Lasota J. Gastrointestinal stromal tumors. Gastroenterol Clin North Am. 2013; 42:399–415.
Article8. Soft tissue sarcoma, version 1.2018. National Comprehensive Cancer Network: Clinical Practice Guidelines in Oncology [Internet]. Plymouth Meeting: National Comprehensive Cancer Network;2018. [cited 2017 Dec 14]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf.9. Agaram NP, Wong GC, Guo T, et al. Novel V600E BRAF mutations in imatinib-naive and imatinib-resistant gastrointestinal stromal tumors. Genes Chromosomes Cancer. 2008; 47:853–9.10. Gaal J, Stratakis CA, Carney JA, et al. SDHB immunohistochemistry: a useful tool in the diagnosis of Carney-Stratakis and Carney triad gastrointestinal stromal tumors. Mod Pathol. 2011; 24:147–51.11. Patrikidou A, Domont J, Chabaud S, et al. Long-term outcome of molecular subgroups of GIST patients treated with standard-dose imatinib in the BFR14 trial of the French Sarcoma Group. Eur J Cancer. 2016; 52:173–80.
Article12. Heinrich MC, Owzar K, Corless CL, et al. Correlation of kinase genotype and clinical outcome in the North American Intergroup Phase III Trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: CALGB 150105 Study by Cancer and Leukemia Group B and Southwest Oncology Group. J Clin Oncol. 2008; 26:5360–7.
Article13. Lasota J, Miettinen M. Clinical significance of oncogenic KIT and PDGFRA mutations in gastrointestinal stromal tumours. Histopathology. 2008; 53:245–66.14. Heinrich MC, Rankin C, Blanke CD, et al. Correlation of long-term results of imatinib in advanced gastrointestinal stromal tumors with next-generation sequencing results: analysis of phase 3 SWOG intergroup trial S0033. JAMA Oncol. 2017; 3:944–52.15. Heinrich MC, Maki RG, Corless CL, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol. 2008; 26:5352–9.
Article16. Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008; 39:1411–9.
Article17. Hu TH, Chuah SK, Lin JW, et al. Expression and prognostic role of molecular markers in 99 KIT-positive gastric stromal tumors in Taiwanese. World J Gastroenterol. 2006; 12:595–602.18. Nakamura N, Yamamoto H, Yao T, et al. Prognostic significance of expressions of cell-cycle regulatory proteins in gastrointestinal stromal tumor and the relevance of the risk grade. Hum Pathol. 2005; 36:828–37.
Article19. Vu HA, Xinh PT, Kikushima M, et al. A recurrent duodenal gastrointestinal stromal tumor with a frameshift mutation resulting in a stop codon in KIT exon 13. Genes Chromosomes Cancer. 2005; 42:179–83.20. Chiang NJ, Chen LT, Tsai CR, Chang JS. The epidemiology of gastrointestinal stromal tumors in Taiwan, 1998-2008: a nation-wide cancer registry-based study. BMC Cancer. 2014; 14:102.
Article21. Brabec P, Sufliarsky J, Linke Z, et al. A whole population study of gastrointestinal stromal tumors in the Czech Republic and Slovakia. Neoplasma. 2009; 56:459–64.
Article22. Tryggvason G, Gíslason HG, Magnússon MK, Jónasson JG. Gastrointestinal stromal tumors in Iceland, 1990-2003: the icelandic GIST study, a population-based incidence and pathologic risk stratification study. Int J Cancer. 2005; 117:289–93.
Article23. Yamamoto H, Oda Y, Kawaguchi K, et al. c-KIT and PDGFRA mutations in extragastrointestinal stromal tumor (gastrointestinal stromal tumor of the soft tissue). Am J Surg Pathol. 2004; 28:479–88.24. Miettinen M, Felisiak-Golabek A, Wang Z, Inaguma S, Lasota J. GIST manifesting as a retroperitoneal tumor: clinicopathologic immunohistochemical, and molecular genetic study of 112 cases. Am J Surg Pathol. 2017; 41:577–85.25. Belev B, Brcˇic´ I, Prejac J, et al. Role of Ki-67 as a prognostic factor in gastrointestinal stromal tumors. World J Gastroenterol. 2013; 19:523–7.
Article26. Ohdaira H, Ohyama S, Yamaguchi T, Yanagisawa A, Kato Y, Urashima M. Ki67 and tumor size as prognostic factors of gastrointestinal stromal tumors. Japan Med Assoc J. 2005; 48:586–92.27. Kandoth C, McLellan MD, Vandin F, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013; 502:333–9.
Article28. Pauser U, Schmedt Auf der Günne N, Klöppel G, Merz H, Feller AC. P53 expression is significantly correlated with high risk of malignancy and epithelioid differentiation in GISTs: an immunohistochemical study of 104 cases. BMC Cancer. 2008; 8:204.
Article29. Corless CL, Heinrich MC. Molecular pathobiology of gastrointestinal stromal sarcomas. Annu Rev Pathol. 2008; 3:557–86.
Article30. Tzen CY, Wang MN, Mau BL. Spectrum and prognostication of KIT and PDGFRA mutation in gastrointestinal stromal tumors. Eur J Surg Oncol. 2008; 34:563–8.31. Joensuu H, Wardelmann E, Sihto H, et al. Effect of KIT and PDGFRA mutations on survival in patients with gastrointestinal stromal tumors treated with adjuvant imatinib: an exploratory analysis of a randomized clinical trial. JAMA Oncol. 2017; 3:602–9.32. Corless CL, Ballman KV, Antonescu CR, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol. 2014; 32:1563–70.
Article33. Lasota J, Corless CL, Heinrich MC, et al. Clinicopathologic profile of gastrointestinal stromal tumors (GISTs) with primary KIT exon 13 or exon 17 mutations: a multicenter study on 54 cases. Mod Pathol. 2008; 21:476–84.34. Garner AP, Gozgit JM, Anjum R, et al. Ponatinib inhibits polyclonal drug-resistant KIT oncoproteins and shows therapeutic potential in heavily pretreated gastrointestinal stromal tumor (GIST) patients. Clin Cancer Res. 2014; 20:5745–55.35. Gao J, Tian Y, Li J, Sun N, Yuan J, Shen L. Secondary mutations of c-KIT contribute to acquired resistance to imatinib and decrease efficacy of sunitinib in Chinese patients with gastrointestinal stromal tumors. Med Oncol. 2013; 30:522.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gastrointestinal Stromal Tumors; Clinicopathological Features and Prognostic Factors
- A case of huge Gastrointestinal stromal tumor masquerading as an ovarian malignancy
- Comparative Study of the Clinicopathologic Characteristics between Gastrointestinal Stromal Tumors Arising from the Stomach and Small Bowel
- Expression and Clinicopathological Significance of CD9 in Gastrointestinal Stromal Tumor
- The Role of Endoscopic Ultrasonography in Differentiating Benign and Malignant Stromal Tumors of Upper Gastrointestinal Tract