J Korean Med Sci.
2013 Oct;28(10):1443-1448. 10.3346/jkms.2013.28.10.1443.
Expression and Clinicopathological Significance of CD9 in Gastrointestinal Stromal Tumor
- Affiliations
-
- 1Department of Gastrointestinal Surgery, West China Hospital, Sichuan University, Chengdu, Sichuan Province, China. hxwcwk@126.com
- 2Department of Gastrointestinal Surgery of the Affiliated Hospital of Guiyang Medical University, Guiyang, Guizhou Province, China.
- KMID: 1777679
- DOI: http://doi.org/10.3346/jkms.2013.28.10.1443
Abstract
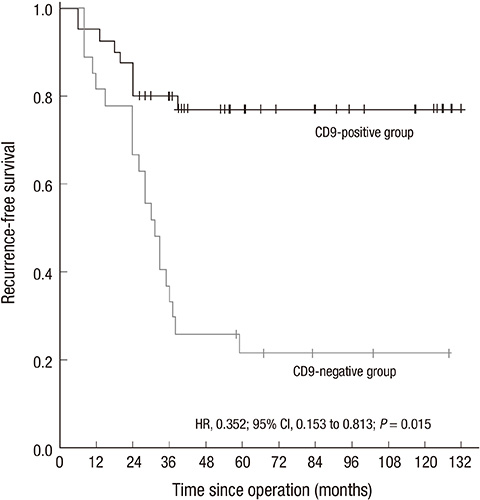
- This study investigated the expression and clinicopathological significance of CD9 in gastrointestinal stromal tumor (GIST). Immunohistochemistry staining for CD9 was performed on tumor tissues from 74 GIST patients. The correlation with clinicopathological features, risk classification and prognosis was analyzed. CD9-positive staining comprised 59.5% (44/74) of the GIST patients. The CD9-positive expression rate of the sample was significantly associated with diameter (P = 0.028), mitotic counts (P = 0.035), risk classification (P = 0.018) and three-year recurrence-free survival (RFS) (P < 0.001). Cox proportional hazards regression (HR = 0.352; P = 0.015) showed that CD9 is an independent factor for post-operative RFS. The subgroup analysis showed that CD9 expression in gastric stromal tumor (GST) is significantly associated with diameter (P = 0.031), risk classification (P = 0.023) and three-year RFS (P = 0.001). The Cox proportional hazards regression (HR = 0.104; P = 0.006) also showed that CD9 is an independent factor for RFS of GST. However, CD9 expression does not have a statistically significant correlation with clinicopathological features, risk classification, and prognosis in non-GST. In conclusion, CD9 expression in GIST appears to be associated with the recurrence and/or metastasis of GIST patients, especially in GST, which may indicate the important role of CD9 in the malignant biological behavior and prognosis of GST.
Keyword
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Antigens, CD9/*genetics/*metabolism
Disease-Free Survival
Female
Gastrointestinal Neoplasms/metabolism/mortality/*pathology
Gastrointestinal Stromal Tumors/metabolism/mortality/*pathology
*Gene Expression Regulation, Neoplastic
Humans
Immunohistochemistry
Kaplan-Meier Estimate
Male
Middle Aged
Prognosis
Proportional Hazards Models
Risk Factors
Antigens, CD9
Figure
Reference
-
1. Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008; 39:1411–1419.2. Buim ME, Lourenço SV, Carvalho KC, Cardim R, Pereira C, Carvalho AL, Fregnani JH, Soares FA. Downregulation of CD9 protein expression is associated with aggressive behavior of oral squamous cell carcinoma. Oral Oncol. 2010; 46:166–171.3. Nakazawa Y, Sato S, Naito M, Kato Y, Mishima K, Arai H, Tsuruo T, Fujita N. Tetraspanin family member CD9 inhibits Aggrus/podoplanin-induced platelet aggregation and suppresses pulmonary metastasis. Blood. 2008; 112:1730–1739.4. Yao JC, Wang L, Wei D, Gong W, Hassan M, Wu TT, Mansfield P, Ajani J, Xie K. Association between expression of transcription factor Sp1 and increased vascular endothelial growth factor expression, advanced stage, and poor survival in patients with resected gastric cancer. Clin Cancer Res. 2004; 10:4109–4117.5. Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, Pisters PW, Raut CP, Riedel RF, Schuetze S, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010; 8:S1–S41.6. Hassan I, You YN, Shyyan R, Dozois EJ, Smyrk TC, Okuno SH, Schleck CD, Hodge DO, Donohue JH. Surgically managed gastrointestinal stromal tumors: a comparative and prognostic analysis. Ann Surg Oncol. 2008; 15:52–59.7. Kang YK, Kim KM, Sohn T, Choi D, Kang HJ, Ryu MH, Kim WH, Yang HK. Clinical practice guideline for accurate diagnosis and effective treatment of gastrointestinal stromal tumor in Korea. J Korean Med Sci. 2010; 25:1543–1552.8. Wang WL, Conley A, Reynoso D, Nolden L, Lazar AJ, George S, Trent JC. Mechanisms of resistance to imatinib and sunitinib in gastrointestinal stromal tumor. Cancer Chemother Pharmacol. 2011; 67:S15–S24.9. Breiner JA, Meis-Kindblom J, Kindblom LG, McComb E, Liu J, Nelson M, Bridge JA. Loss of 14q and 22q in gastrointestinal stromal tumors (pacemaker cell tumors). Cancer Genet Cytogenet. 2000; 120:111–116.10. Chen Y, Liou CP, Tseng HH, Jan YJ, Li CF, Tzeng CC. Deletions of chromosome 1p and 15q are associated with aggressiveness of gastrointestinal stromal tumors. J Formos Med Assoc. 2009; 108:28–37.11. Yang J, Du X, Lazar AJ, Pollock R, Hunt K, Chen K, Hao X, Trent J, Zhang W. Genetic aberrations of gastrointestinal stromal tumors. Cancer. 2008; 113:1532–1543.12. Okamoto Y, Sawaki A, Ito S, Nishida T, Takahashi T, Toyota M, Suzuki H, Shinomura Y, Takeuchi I, Shinjo K, et al. Aberrant DNA methylation associated with aggressiveness of gastrointestinal stromal tumour. Gut. 2012; 61:392–401.13. Huang CL, Liu D, Masuya D, Kameyama K, Nakashima T, Yokomise H, Ueno M, Miyake M. MRP-1/CD9 gene transduction downregulates Wnt signal pathways. Oncogene. 2004; 23:7475–7483.14. Imhof I, Gasper WJ, Derynck R. Association of tetraspanin CD9 with transmembrane TGF{alpha} confers alterations in cell-surface presentation of TGF{alpha} and cytoskeletal organization. J Cell Sci. 2008; 121:2265–2274.15. Saito Y, Tachibana I, Takeda Y, Yamane H, He P, Suzuki M, Minami S, Kijima T, Yoshida M, Kumagai T, et al. Absence of CD9 enhances adhesion-dependent morphologic differentiation, survival, and matrix metalloproteinase-2 production in small cell lung cancer cells. Cancer Res. 2006; 66:9557–9565.16. Soyuer S, Soyuer I, Unal D, Ucar K, Yildiz OG, Orhan O. Prognostic significance of CD9 expression in locally advanced gastric cancer treated with surgery and adjuvant chemoradiotherapy. Pathol Res Pract. 2010; 206:607–610.17. Kohmo S, Kijima T, Otani Y, Mori M, Minami T, Takahashi R, Nagatomo I, Takeda Y, Kida H, Goya S, et al. Cell surface tetraspanin CD9 mediates chemoresistance in small cell lung cancer. Cancer Res. 2010; 70:8025–8035.18. Mhawech P, Herrmann F, Coassin M, Guillou L, Iselin CE. Motility-related protein 1 (MRP-1/CD9) expression in urothelial bladder carcinoma and its relation to tumor recurrence and progression. Cancer. 2003; 98:1649–1657.19. Setoguchi T, Kikuchi H, Yamamoto M, Baba M, Ohta M, Kamiya K, Tanaka T, Baba S, Goto-Inoue N, Setou M, et al. Microarray analysis identifies versican and CD9 as potent prognostic markers in gastric gastrointestinal stromal tumors. Cancer Sci. 2011; 102:883–889.20. Fan J, Zhu GZ, Niles RM. Expression and function of CD9 in melanoma cells. Mol Carcinog. 2010; 49:85–93.21. Zou Q, Xiong L, Yang Z, Lv F, Yang L, Miao X. Expression levels of HMGA2 and CD9 and its clinicopathological significances in the benign and malignant lesions of the gallbladder. World J Surg Oncol. 2012; 10:92.22. Zöller M. Tetraspanins: push and pull in suppressing and promoting metastasis. Nat Rev Cancer. 2009; 9:40–55.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- CD9 Expression in Colorectal Carcinomas and Its Prognostic Significance
- CD9 Expression in Tumor Cells Is Associated with Poor Prognosis in Patients with Invasive Lobular Carcinoma
- Extra-gastrointestinal Stromal Tumor on the Inner Urinary Bladder Wall
- A Case of Massive Bleeding from Jejunal Stromal Tumor Diagnosed by Intraoperative Enteroscopy: A Case of Jejunal Stromal Tumor Bleeding
- Extragastrointestinal Stromal Tumor Mimicking Gastric Subepithelial Tumor