World J Mens Health.
2020 Jan;38(1):85-94. 10.5534/wjmh.190030.
Cross-Sectional Association of Metabolic Syndrome and Its Components with Serum Testosterone Levels in a Korean-Screened Population
- Affiliations
-
- 1Department of Urology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 2Department of Health Screening and Promotion Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Department of Urology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. tyahn@amc.seoul.kr
- KMID: 2465413
- DOI: http://doi.org/10.5534/wjmh.190030
Abstract
- PURPOSE
We evaluated the associations of metabolic syndrome (MetS) and its components with testosterone levels in the Korean population.
MATERIALS AND METHODS
This cross-sectional study was performed among 6,967 adult (age≥20 years) men who attended health screening during 2006 to 2015. MetS was defined using the National Cholesterol Education Program Adult Treatment Panel III criteria. Associations were evaluated using unconditional logistic regression.
RESULTS
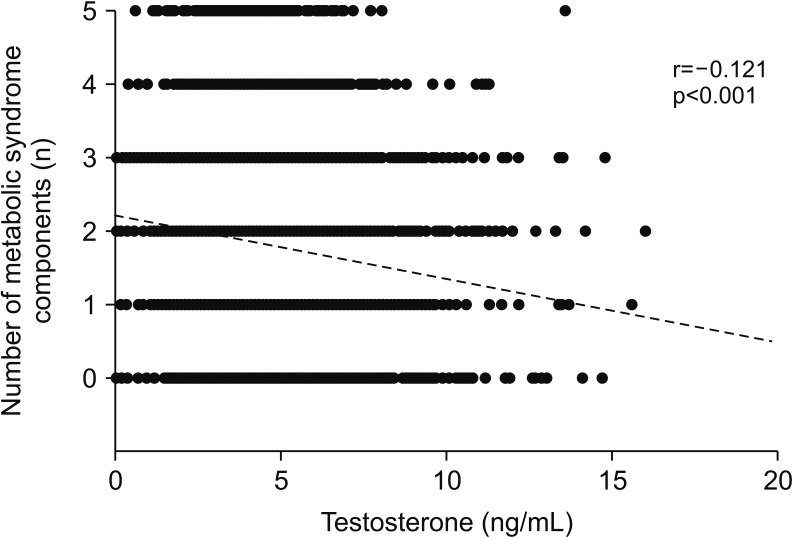
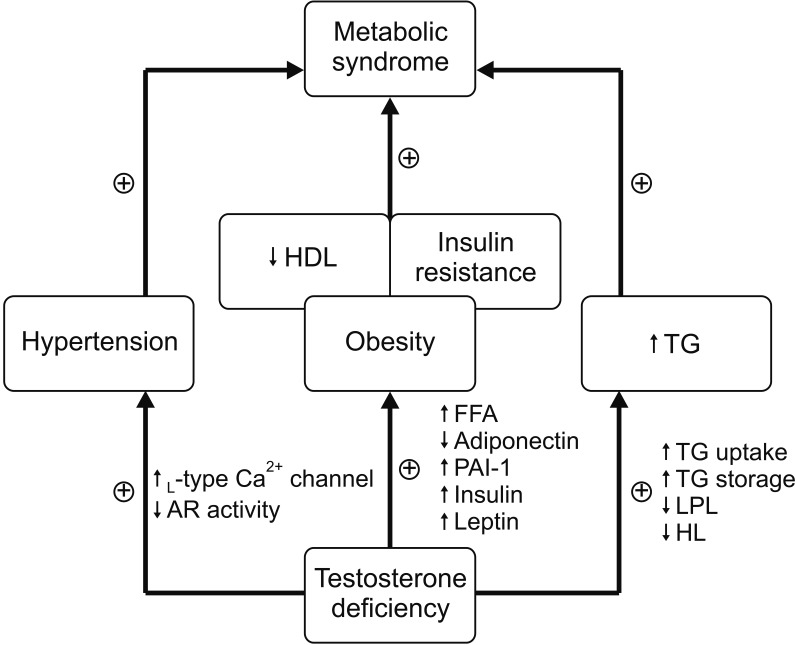
The estimated age-adjusted prevalence of MetS in adult and middle-aged (≥40 years) Korean men was 27.5% and 30.6%, respectively. Quartile analysis showed that high serum testosterone levels were significantly associated with a low risk of MetS (highest vs. lowest quartile, odds ratio=0.528; p(trend)<0.001), with an approximately 13% reduction in MetS risk per 1 ng/mL increment of serum testosterone levels. After considering covariates such as age and body mass index (BMI), the reduction in MetS risk was attenuated but remained significant (7% reduced risk per 1 ng/mL). Testosterone levels were inversely correlated with all MetS components, including hyperglycemia (r=−0.041), increased body size (r=−0.093), increased triglyceride levels (r=−0.090), decreased high-density lipoprotein cholesterol levels (r=−0.030), and elevated blood pressure (r=−0.071, all p<0.05). Among them, elevated triglyceride levels and blood pressure were independently associated with low serum testosterone levels, even after adjustment for age and BMI.
CONCLUSIONS
Serum testosterone levels were inversely associated with MetS in Korean men. This association was attenuated after adjustment for age and BMI but remained significant. Among MetS components, increased triglyceride levels and elevated blood pressure were independently associated with testosterone levels, regardless of obesity.
Keyword
MeSH Terms
Figure
Reference
-
1. Isomaa B, Almgren P, Tuomi T, Forsén B, Lahti K, Nissén M, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001; 24:683–689. PMID: 11315831.
Article2. Stern MP, Williams K, González-Villalpando C, Hunt KJ, Haffner SM. Does the metabolic syndrome improve identification of individuals at risk of type 2 diabetes and/or cardiovascular disease? Diabetes Care. 2004; 27:2676–2681. PMID: 15505004.
Article3. Mozumdar A, Liguori G. Persistent increase of prevalence of metabolic syndrome among U.S. adults: NHANES III to NHANES 1999–2006. Diabetes Care. 2011; 34:216–219. PMID: 20889854.
Article4. Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA. 2015; 313:1973–1974. PMID: 25988468.
Article5. Nestel P, Lyu R, Low LP, Sheu WH, Nitiyanant W, Saito I, et al. Metabolic syndrome: recent prevalence in East and Southeast Asian populations. Asia Pac J Clin Nutr. 2007; 16:362–367. PMID: 17468095.6. Lim S, Shin H, Song JH, Kwak SH, Kang SM, Won Yoon J, et al. Increasing prevalence of metabolic syndrome in Korea: the Korean National Health and Nutrition Examination Survey for 1998–2007. Diabetes Care. 2011; 34:1323–1328. PMID: 21505206.7. Tsai EC, Matsumoto AM, Fujimoto WY, Boyko EJ. Association of bioavailable, free, and total testosterone with insulin resistance: influence of sex hormone-binding globulin and body fat. Diabetes Care. 2004; 27:861–868. PMID: 15047639.8. Stellato RK, Feldman HA, Hamdy O, Horton ES, McKinlay JB. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts male aging study. Diabetes Care. 2000; 23:490–494. PMID: 10857940.
Article9. Fogari R, Preti P, Zoppi A, Fogari E, Rinaldi A, Corradi L, et al. Serum testosterone levels and arterial blood pressure in the elderly. Hypertens Res. 2005; 28:625–630. PMID: 16392765.
Article10. Khaw KT, Dowsett M, Folkerd E, Bingham S, Wareham N, Luben R, et al. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) prospective population study. Circulation. 2007; 116:2694–2701. PMID: 18040028.11. Laaksonen DE, Niskanen L, Punnonen K, Nyyssönen K, Tuomainen TP, Salonen R, et al. Sex hormones, inflammation and the metabolic syndrome: a population-based study. Eur J Endocrinol. 2003; 149:601–608. PMID: 14641004.
Article12. Muller M, Grobbee DE, den Tonkelaar I, Lamberts SW, van der Schouw YT. Endogenous sex hormones and metabolic syndrome in aging men. J Clin Endocrinol Metab. 2005; 90:2618–2623. PMID: 15687322.
Article13. Laaksonen DE, Niskanen L, Punnonen K, Nyyssönen K, Tuomainen TP, Valkonen VP, et al. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004; 27:1036–1041. PMID: 15111517.
Article14. Kupelian V, Page ST, Araujo AB, Travison TG, Bremner WJ, McKinlay JB. Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese men. J Clin Endocrinol Metab. 2006; 91:843–850. PMID: 16394089.
Article15. Brand JS, Rovers MM, Yeap BB, Schneider HJ, Tuomainen TP, Haring R, et al. Testosterone, sex hormone-binding globulin and the metabolic syndrome in men: an individual participant data meta-analysis of observational studies. PLoS One. 2014; 9:e100409. PMID: 25019163.
Article16. Akishita M, Fukai S, Hashimoto M, Kameyama Y, Nomura K, Nakamura T, et al. Association of low testosterone with metabolic syndrome and its components in middle-aged Japanese men. Hypertens Res. 2010; 33:587–591. PMID: 20339372.
Article17. Tong PC, Ho CS, Yeung VT, Ng MC, So WY, Ozaki R, et al. Association of testosterone, insulin-like growth factor-I, and C-reactive protein with metabolic syndrome in Chinese middle-aged men with a family history of type 2 diabetes. J Clin Endocrinol Metab. 2005; 90:6418–6423. PMID: 16189249.
Article18. Kyung YS, You D, Jeong IG, Han S, Kim HK, Kim CS. Changes in weight and metabolic syndrome are associated with prostate growth rate over a 5-year period. Urology. 2017; 103:185–190. PMID: 27720970.
Article19. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005; 112:2735–2752. PMID: 16157765.20. Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009; 120:1640–1645. PMID: 19805654.21. KOrean Statistical Information Service (KOSIS). Korean population census 2010 [Internet]. Daejeon: Statistics Korea;c2010. cited 2019 Jan 15. Available from: http://kosis.kr/eng/index/index.do.22. Kwon HS, Park YM, Lee HJ, Lee JH, Choi YH, Ko SH, et al. Prevalence and clinical characteristics of the metabolic syndrome in middle-aged Korean adults. Korean J Intern Med. 2005; 20:310–316. PMID: 16491829.
Article23. Tsujimura A, Miyagawa Y, Takezawa K, Okuda H, Fukuhara S, Kiuchi H, et al. Is low testosterone concentration a risk factor for metabolic syndrome in healthy middle-aged men? Urology. 2013; 82:814–819. PMID: 24074976.
Article24. Rebuffé-Scrive M, Mårin P, Björntorp P. Effect of testosterone on abdominal adipose tissue in men. Int J Obes. 1991; 15:791–795. PMID: 1778664.25. Torkler S, Wallaschofski H, Baumeister SE, Völzke H, Dörr M, Felix S, et al. Inverse association between total testosterone concentrations, incident hypertension and blood pressure. Aging Male. 2011; 14:176–182. PMID: 21087174.
Article26. Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004; 286:R233–R249. PMID: 14707008.
Article27. Hall J, Jones RD, Jones TH, Channer KS, Peers C. Selective inhibition of L-type Ca2+ channels in A7r5 cells by physiological levels of testosterone. Endocrinology. 2006; 147:2675–2680. PMID: 16527846.
Article28. Jackson RL, Yates MT, McNerney CA, Kashyap ML. Relationship between post-heparin plasma lipases, triglycerides and high density lipoproteins in normal subjects. Horm Metab Res. 1990; 22:289–294. PMID: 2347543.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Associations of Metabolic Syndrome with Total Testosterone and Homocysteine Levels in Male Korean Workers
- Testosterone and metabolic syndrome in men
- Seasonal Variations and Correlations between Vitamin D and Total Testosterone Levels
- The Association Between Serum Albumin Levels and Metabolic Syndrome in a Rural Population of Korea
- Relationship between Serum TSH Level within the Normal Reference Range and the Metabolic Syndrome