Cancer Res Treat.
2019 Apr;51(2):832-840. 10.4143/crt.2018.311.
Dynamics of Soluble Programmed Death-Ligand 1 (sPDL1) during Chemotherapy and Its Prognostic Implications in Cancer Patients: Biomarker Development in Immuno-oncology
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea. ohdoyoun@snu.ac.kr
- 2Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2464428
- DOI: http://doi.org/10.4143/crt.2018.311
Abstract
- PURPOSE
The soluble programmed death-ligand 1 (sPDL1) has immunosuppressive activity and is a candidate biomarker for immuno-oncology drug development. In this study, we measured sPDL1 at pre- and post-chemotherapy and at disease progression to uncover the dynamics of sPDL1 during treatment in biliary tract cancer (BTC) patients.
MATERIALS AND METHODS
From 90 BTC patients (training cohort, 53; validation cohort, 37) who were candidates for palliative first-line chemotherapy, blood was collected at pre- and post-chemotherapy (at the time of best response) and at disease progression. The sPDL1 levels were measured using an enzyme-linked immunosorbent assay. Responses to chemotherapy, overall survival (OS), and other prognostic factors including the neutrophil-lymphocyte ratio (NLR) were analyzed.
RESULTS
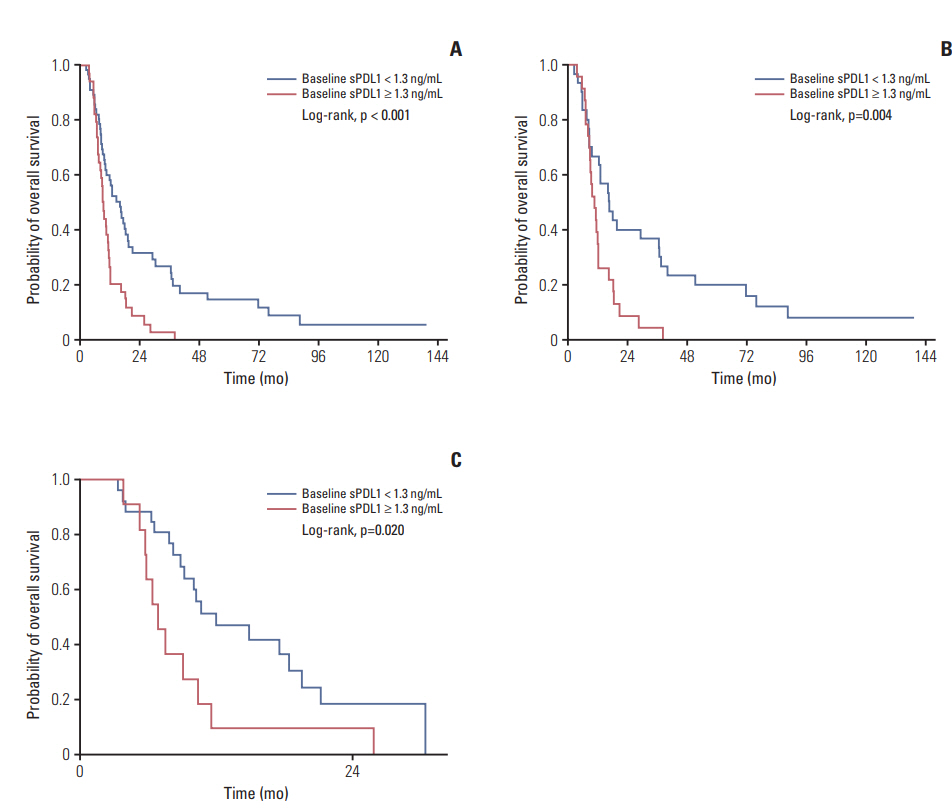
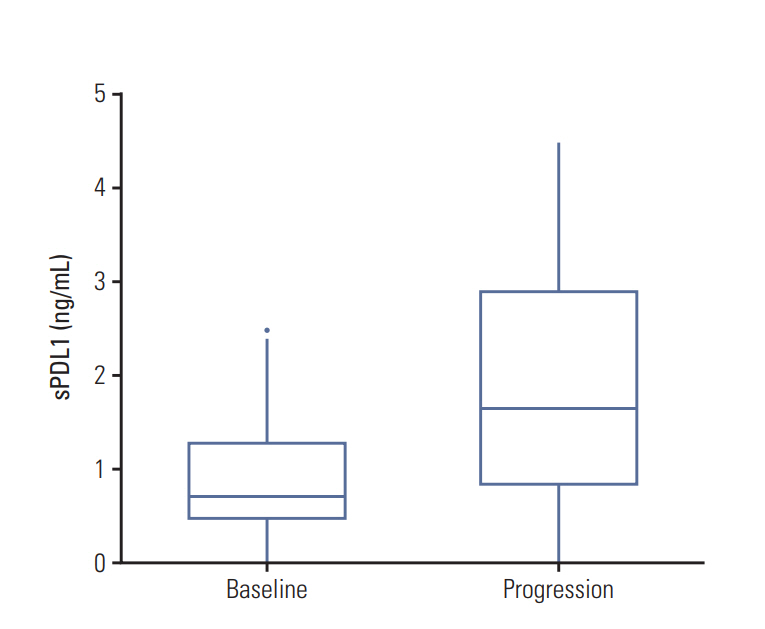
The OS of all patients was 11.5 months (confidence interval [CI], 9.7 to 16.2). The best response was complete response in seven (7.8%), partial response in 20 (22.2%), stable disease in 52 (57.8%), and disease progression (PD) in 11 patients (12.2%). Patients with high pre-chemotherapy sPDL1 (≥ 1.30 ng/mL) showed worse OS than patients with low prechemotherapy sPDL1 (9.1 months vs. 12.5 months, p=0.003). In multivariate analyses, high pre-chemotherapy sPDL1 (hazard ratio [HR], 1.96; 95% CI, 1.2 to 3.9; p=0.011) and high pre-chemotherapy NLR (HR, 1.82; 95% CI, 1.1 to 3.0; p=0.020) were independent poor prognostic factors for OS. At the time of PD, sPDL1 was increased significantly compared with pre-chemotherapy sPDL1 (1.59 ng/mL vs. 0.72 ng/mL, p=0.003).
CONCLUSION
The sPDL1 at pre-chemotherapy confers the prognostic value for OS in BTC patients under palliative chemotherapy. The dynamics of sPDL1 during chemotherapy correlate with disease burden and have prognostic value.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Prognostic Value of Serum Soluble Programmed Death-Ligand 1 and Dynamics During Chemotherapy in Advanced Gastric Cancer Patients
Woochan Park, Ju-Hee Bang, Ah-Rong Nam, Mei Hua Jin, Hyerim Seo, Jae-Min Kim, Kyoung Seok Oh, Tae-Yong Kim, Do-Youn Oh
Cancer Res Treat. 2021;53(1):199-206. doi: 10.4143/crt.2020.497.
Reference
-
References
1. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012; 12:252–64.
Article2. Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015; 161:205–14.
Article3. Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015; 33:1974–82.
Article4. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015; 372:2509–20.5. Chen J, Jiang CC, Jin L, Zhang XD. Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann Oncol. 2016; 27:409–16.
Article6. Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy: inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res. 2012; 18:6580–7.7. Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010; 28:3167–75.
Article8. Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007; 13:2151–7.
Article9. Sasada T, Suekane S. Variation of tumor-infiltrating lymphocytes in human cancers: controversy on clinical significance. Immunotherapy. 2011; 3:1235–51.
Article10. Nakakubo Y, Miyamoto M, Cho Y, Hida Y, Oshikiri T, Suzuoki M, et al. Clinical significance of immune cell infiltration within gallbladder cancer. Br J Cancer. 2003; 89:1736–42.
Article11. Goeppert B, Frauenschuh L, Zucknick M, Stenzinger A, Andrulis M, Klauschen F, et al. Prognostic impact of tumourinfiltrating immune cells on biliary tract cancer. Br J Cancer. 2013; 109:2665–74.
Article12. Chai Y. Immunotherapy of biliary tract cancer. Tumour Biol. 2016; 37:2817–21.
Article13. Ha H, Nam AR, Bang JH, Park JE, Kim TY, Lee KH, et al. Soluble programmed death-ligand 1 (sPDL1) and neutrophilto-lymphocyte ratio (NLR) predicts survival in advanced biliary tract cancer patients treated with palliative chemotherapy. Oncotarget. 2016; 7:76604–12.
Article14. Frigola X, Inman BA, Lohse CM, Krco CJ, Cheville JC, Thompson RH, et al. Identification of a soluble form of B7-H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin Cancer Res. 2011; 17:1915–23.
Article15. Williams BA, Mandrekar JN, Mandrekar SJ, Cha SS, Furth AF. Finding optimal cutpoints for continuous covariates with binary and time-to-event outcomes. Technical report series No. 79. Rochester, MN: Mayo Foundation;2006.16. Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004; 101:17174–9.
Article17. Park JJ, Omiya R, Matsumura Y, Sakoda Y, Kuramasu A, Augustine MM, et al. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood. 2010; 116:1291–8.
Article18. Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007; 27:111–22.
Article19. Krambeck AE, Dong H, Thompson RH, Kuntz SM, Lohse CM, Leibovich BC, et al. Survivin and b7-h1 are collaborative predictors of survival and represent potential therapeutic targets for patients with renal cell carcinoma. Clin Cancer Res. 2007; 13:1749–56.
Article20. Choueiri TK, Figueroa DJ, Fay AP, Signoretti S, Liu Y, Gagnon R, et al. Correlation of PD-L1 tumor expression and treatment outcomes in patients with renal cell carcinoma receiving sunitinib or pazopanib: results from COMPARZ, a randomized controlled trial. Clin Cancer Res. 2015; 21:1071–7.
Article21. Fukuda T, Kamai T, Masuda A, Nukui A, Abe H, Arai K, et al. Higher preoperative serum levels of PD-L1 and B7-H4 are associated with invasive and metastatic potential and predictable for poor response to VEGF-targeted therapy and unfavorable prognosis of renal cell carcinoma. Cancer Med. 2016; 5:1810–20.22. Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014; 515:563–7.
Article23. Han JJ, Kim DW, Koh J, Keam B, Kim TM, Jeon YK, et al. Change in PD-L1 expression after acquiring resistance to gefitinib in EGFR-mutant non-small-cell lung cancer. Clin Lung Cancer. 2016; 17:263–70.
Article24. Lim SH, Hong M, Ahn S, Choi YL, Kim KM, Oh D, et al. Changes in tumour expression of programmed death-ligand 1 after neoadjuvant concurrent chemoradiotherapy in patients with squamous oesophageal cancer. Eur J Cancer. 2016; 52:1–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prognostic Value of Serum Soluble Programmed Death-Ligand 1 and Dynamics During Chemotherapy in Advanced Gastric Cancer Patients
- An update on immunotherapy with PD-1 and PD-L1 blockade
- Recent Progress in Immunotherapy for Advanced Gastric Cancer
- Gastrointestinal cancer treatment with immune checkpoint inhibitors
- PD-L1 as a Biomarker in Gastric Cancer Immunotherapy