Cancer Res Treat.
2019 Apr;51(2):672-684. 10.4143/crt.2018.137.
Prediction of Acquired Taxane Resistance Using a Personalized Pathway-Based Machine Learning Method
- Affiliations
-
- 1Department of Biochemistry, Konkuk University School of Medicine, Seoul, Korea. palelamp@kku.ac.kr
- KMID: 2464413
- DOI: http://doi.org/10.4143/crt.2018.137
Abstract
- PURPOSE
This study was conducted to develop and validate an individualized prediction model for automated detection of acquired taxane resistance (ATR).
MATERIALS AND METHODS
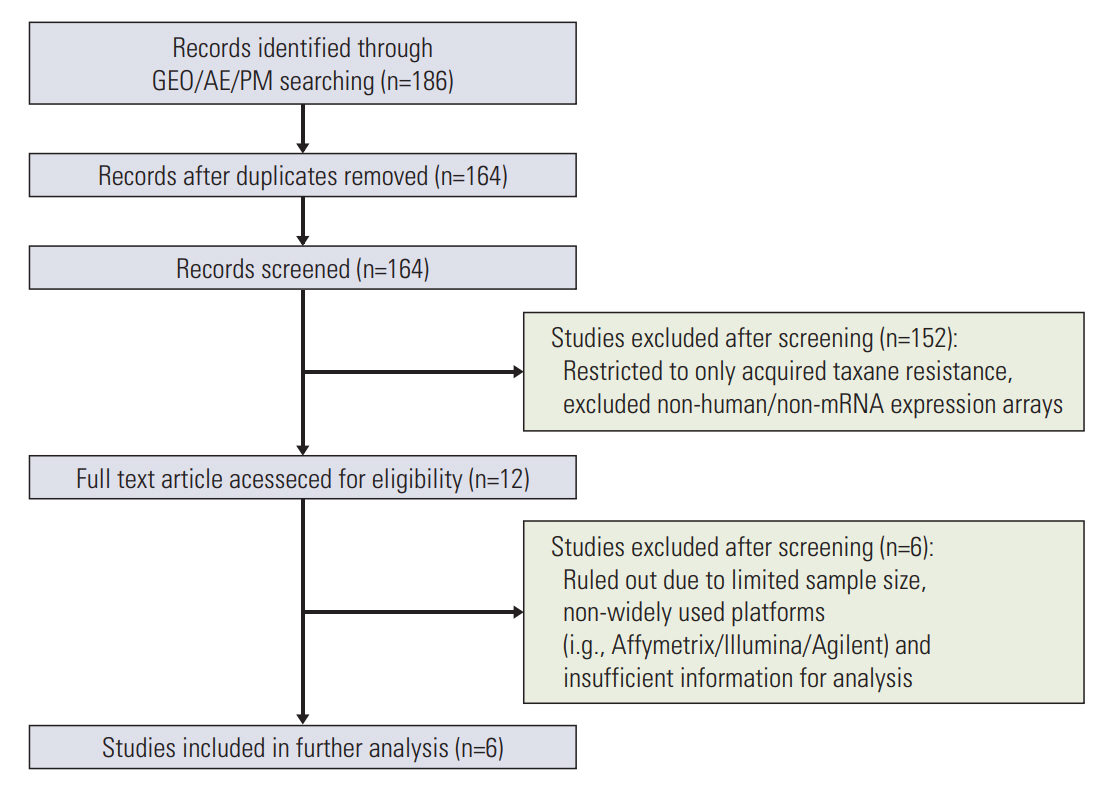
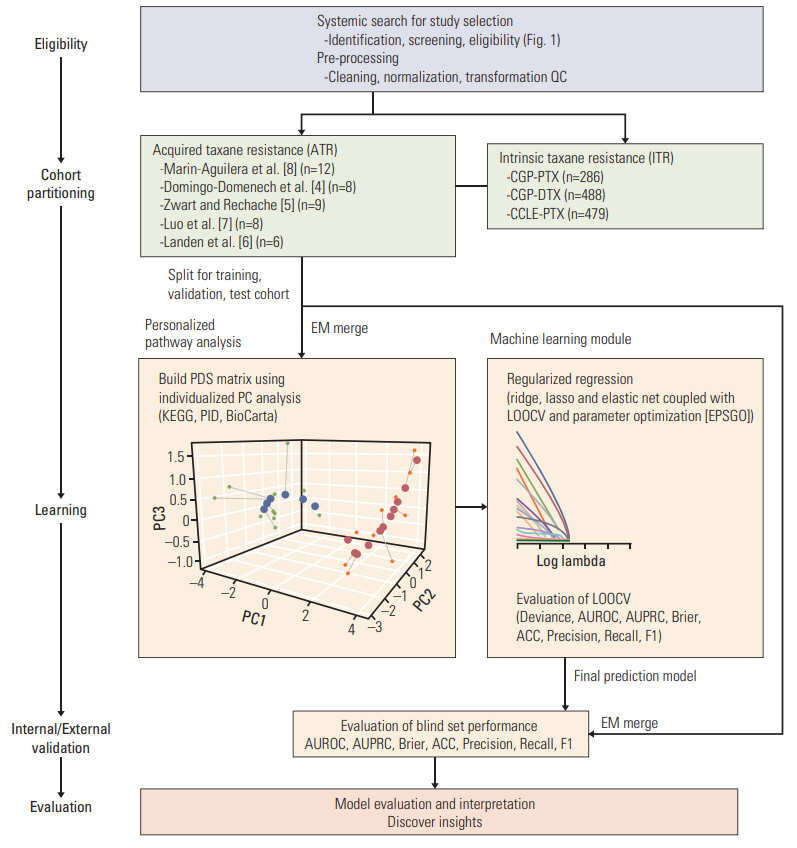
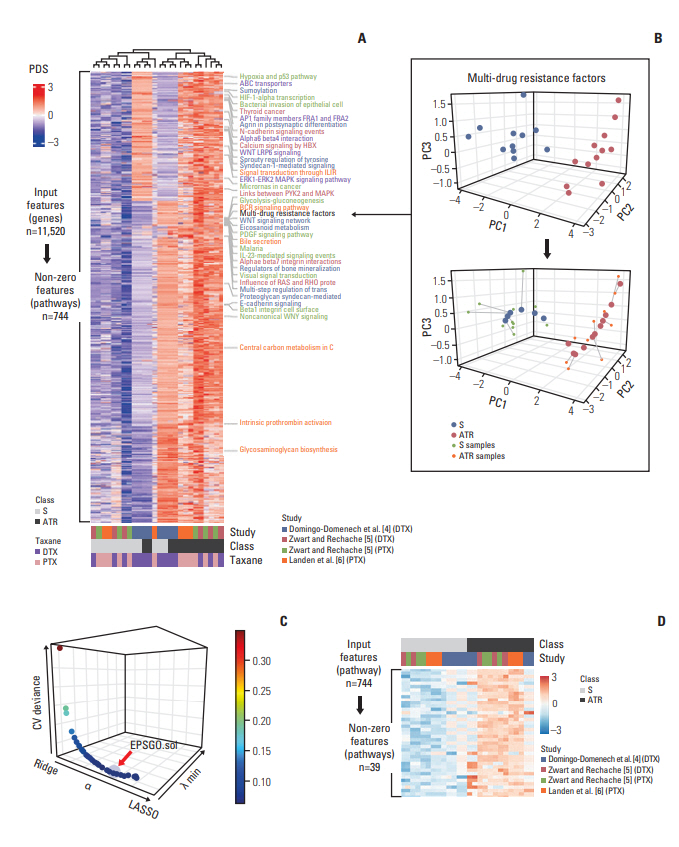
Penalized regression, combinedwith an individualized pathway score algorithm,was applied to construct a predictive model using publically available genomic cohorts of ATR and intrinsic taxane resistance (ITR). To develop a model with enhanced generalizability, we merged multiple ATR studies then updated the learning parameter via robust cross-study validation.
RESULTS
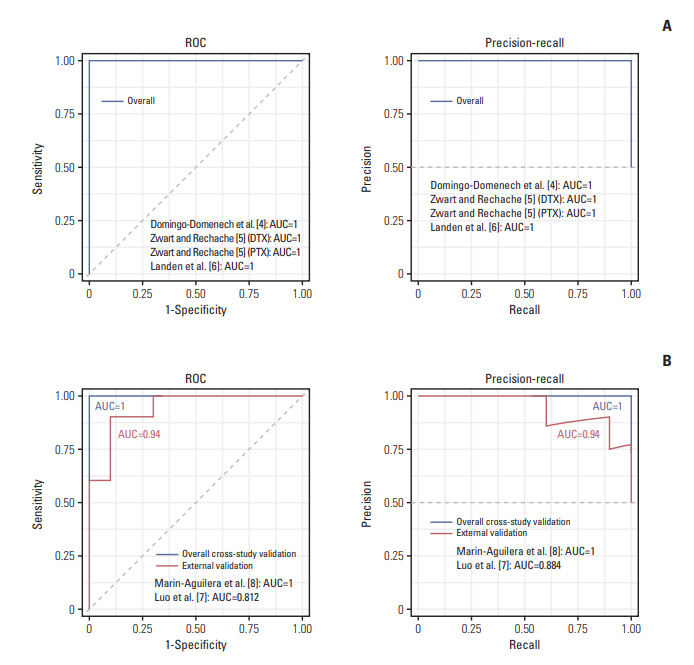
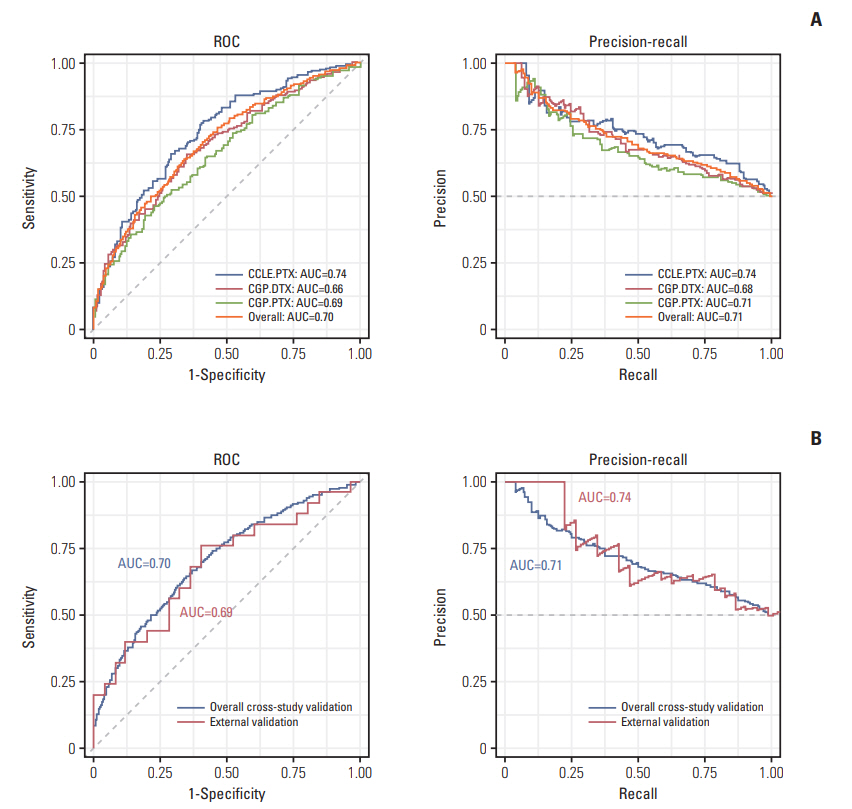
For internal cross-study validation, the ATR model produced a perfect performance with an overall area under the receiver operating curve (AUROC) of 1.000 with an area under the precision-recall curve (AUPRC) of 1.000, a Brier score of 0.007, a sensitivity and a specificity of 100%. The model showed an excellent performance on two independent blind ATR cohorts (overall AUROC of 0.940, AUPRC of 0.940, a Brier score of 0.127). When we applied our algorithm to two large-scale pharmacogenomic resources for ITR, the Cancer Genome Project (CGP) and the Cancer Cell Line Encyclopedia (CCLE), an overall ITR cross-study AUROC was 0.70, which is a far better accuracy than an almost random level reported by previous studies. Furthermore, this model had a high transferability on blind ATR cohorts with an AUROC of 0.69, suggesting that general predictive features may be at work across both ITR and ATR.
CONCLUSION
We successfully constructed a multi-study-derived personalized prediction model for ATR with excellent accuracy, generalizability, and transferability.
MeSH Terms
Figure
Reference
-
References
1. Nussbaumer S, Bonnabry P, Veuthey JL, Fleury-Souverain S. Analysis of anticancer drugs: a review. Talanta. 2011; 85:2265–89.
Article2. Haibe-Kains B, El-Hachem N, Birkbak NJ, Jin AC, Beck AH, Aerts HJ, et al. Inconsistency in large pharmacogenomic studies. Nature. 2013; 504:389–93.
Article3. Bouhaddou M, DiStefano MS, Riesel EA, Carrasco E, Holzapfel HY, Jones DC, et al. Drug response consistency in CCLE and CGP. Nature. 2016; 540:E9–10.
Article4. Domingo-Domenech J, Vidal SJ, Rodriguez-Bravo V, Castillo-Martin M, Quinn SA, Rodriguez-Barrueco R, et al. Suppression of acquired docetaxel resistance in prostate cancer through depletion of notch- and hedgehog-dependent tumorinitiating cells. Cancer Cell. 2012; 22:373–88.
Article5. Zwart A, Rechache N. Gene expression data of sensitive, Docetaxel resistant and paclitaxel resistant MDA-MB-231 cells. GEO DataSets. Series GSE28784 [Internet]. National Center for Biotechnology Information; 2011 [cited 2018 May 2]. Available from: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE28784.6. Landen CN Jr, Goodman B, Katre AA, Steg AD, Nick AM, Stone RL, et al. Targeting aldehyde dehydrogenase cancer stem cells in ovarian cancer. Mol Cancer Ther. 2010; 9:3186–99.
Article7. Luo W, Schork NJ, Marschke KB, Ng SC, Hermann TW, Zhang J, et al. Identification of polymorphisms associated with hypertriglyceridemia and prolonged survival induced by bexarotene in treating non-small cell lung cancer. Anticancer Res. 2011; 31:2303–11.8. Marin-Aguilera M, Codony-Servat J, Kalko SG, Fernandez PL, Bermudo R, Buxo E, et al. Identification of docetaxel resistance genes in castration-resistant prostate cancer. Mol Cancer Ther. 2012; 11:329–39.
Article9. Hughey JJ, Butte AJ. Robust meta-analysis of gene expression using the elastic net. Nucleic Acids Res. 2015; 43:e79.
Article10. Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003; 4:249–64.
Article11. Troyanskaya O, Cantor M, Sherlock G, Brown P, Hastie T, Tibshirani R, et al. Missing value estimation methods for DNA microarrays. Bioinformatics. 2001; 17:520–5.
Article12. Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007; 8:118–27.
Article13. Drier Y, Sheffer M, Domany E. Pathway-based personalized analysis of cancer. Proc Natl Acad Sci U S A. 2013; 110:6388–93.
Article14. Hastie T, Stuetzle W. Principal curves. J Am Stat Assoc. 1989; 84:502–16.
Article15. Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010; 33:1–22.
Article16. Zou H, Hastie T. Regularization and variable selection via the elastic net. J R Stat Soc Series B Stat Methodol. 2005; 67:301–20.
Article17. Sill M, Hielscher T, Becker N, Zucknick M. c060: extended inference with Lasso and elastic-net regularized Cox and generalized linear models. J Stat Softw. 2014; 62:1–22.
Article18. Kuhn M. Building predictive models in R using the caret package. J Stat Softw. 2008; 28:1–26.19. Kanehisa M, Goto S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000; 28:27–30.
Article20. Schaefer CF, Anthony K, Krupa S, Buchoff J, Day M, Hannay T, et al. PID: the pathway interaction database. Nucleic Acids Res. 2009; 37:D674–9.
Article21. Nishimura D. BioCarta. Biotech Softw Internet Rep. 2001; 2:117–20.
Article22. Dong Z, Zhang N, Li C, Wang H, Fang Y, Wang J, et al. Anticancer drug sensitivity prediction in cell lines from baseline gene expression through recursive feature selection. BMC Cancer. 2015; 15:489.
Article23. Greenberger LM, Sampath D. Resistance to taxanes. In : Teicher BA, editor. Cancer drug resistance. Totowa, NJ: Humana Press;2006. p. 329–58.24. Zeng L, Kizaka-Kondoh S, Itasaka S, Xie X, Inoue M, Tanimoto K, et al. Hypoxia inducible factor-1 influences sensitivity to paclitaxel of human lung cancer cell lines under normoxic conditions. Cancer Sci. 2007; 98:1394–401.
Article25. Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006; 5:219–34.
Article26. Seguin L, Desgrosellier JS, Weis SM, Cheresh DA. Integrins and cancer: regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol. 2015; 25:234–40.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Machine Learning vs. Statistical Model for Prediction Modelling: Application in Medical Imaging Research
- Building and analyzing machine learning-based warfarin dose prediction models using scikit-learn
- Machine Learning to Improve the Effectiveness of ANRS in Predicting HIV Drug Resistance
- An improved machine learning model for calculation of intraocular lens power during cataract surgery in Republic of Korea: development
- Artificial intelligence, machine learning, and deep learning in women’s health nursing