Korean Circ J.
2019 Dec;49(12):1183-1195. 10.4070/kcj.2019.0180.
Cardioprotective Potential of an SGLT2 Inhibitor Against Doxorubicin-Induced Heart Failure
- Affiliations
-
- 1Division of Endocrinology and Metabolism, CHA Bundang Medical Center, School of Medicine CHA University, Seongnam, Korea.
- 2Division of Cardiovascular medicine, Department of Internal medicine, Dankook University Hospital, Dankook University School of Medicine, Cheonan, Korea.
- 3Division of Cardiology, National Health Insurance Service Ilsan Hospital, Goyang, Korea.
- 4Graduate School of Medical Science and Engineering, Korea Advanced Institute of Science and Technology, Daejeon, Korea.
- 5Department of Biochemistry, College of Medicine, Catholic Kwandong University, Gangneung, Korea. 49park@cku.ac.kr
- 6Division of Cardiology, Severance Cardiovascular Hospital, Yonsei University College of Medicine, Seoul, Korea. ygko@yuhs.ac
- KMID: 2464299
- DOI: http://doi.org/10.4070/kcj.2019.0180
Abstract
- BACKGROUND AND OBJECTIVES
Recent studies have shown that sodium-glucose co-transporter 2 (SGLT2) inhibitors reduce the risk of heart failure (HF)-associated hospitalization and mortality in patients with diabetes. However, it is not clear whether SGLT2 inhibitors have a cardiovascular benefit in patients without diabetes. We aimed to determine whether empagliflozin (EMPA), an SGLT2 inhibitor, has a protective role in HF without diabetes.
METHODS
Cardiomyopathy was induced in C57BL/6J mice using intraperitoneal injection of doxorubicin (Dox). Mice with HF were fed a normal chow diet (NCD) or an NCD containing 0.03% EMPA. Then we analyzed their phenotypes and performed in vitro experiments to reveal underlying mechanisms of the EMPA's effects.
RESULTS
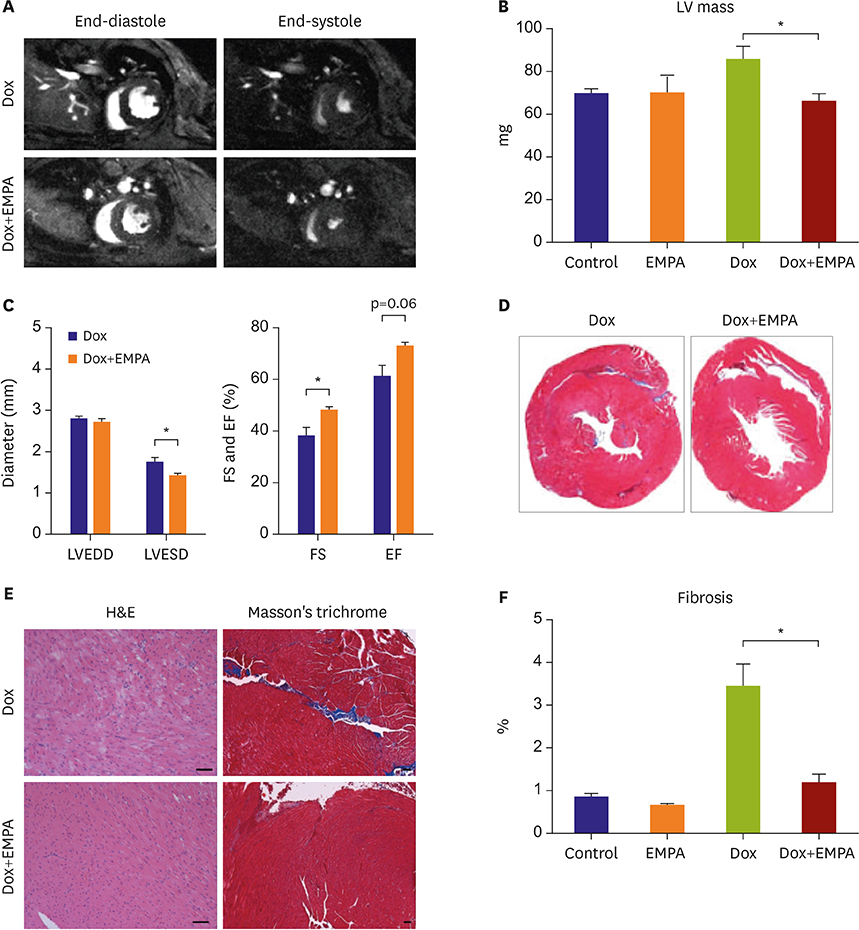
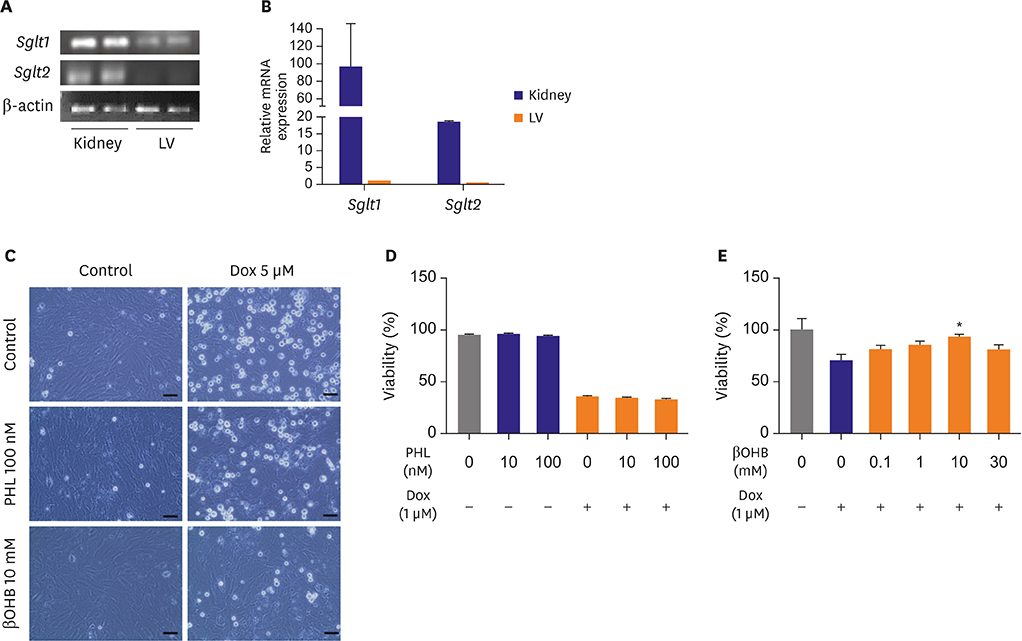
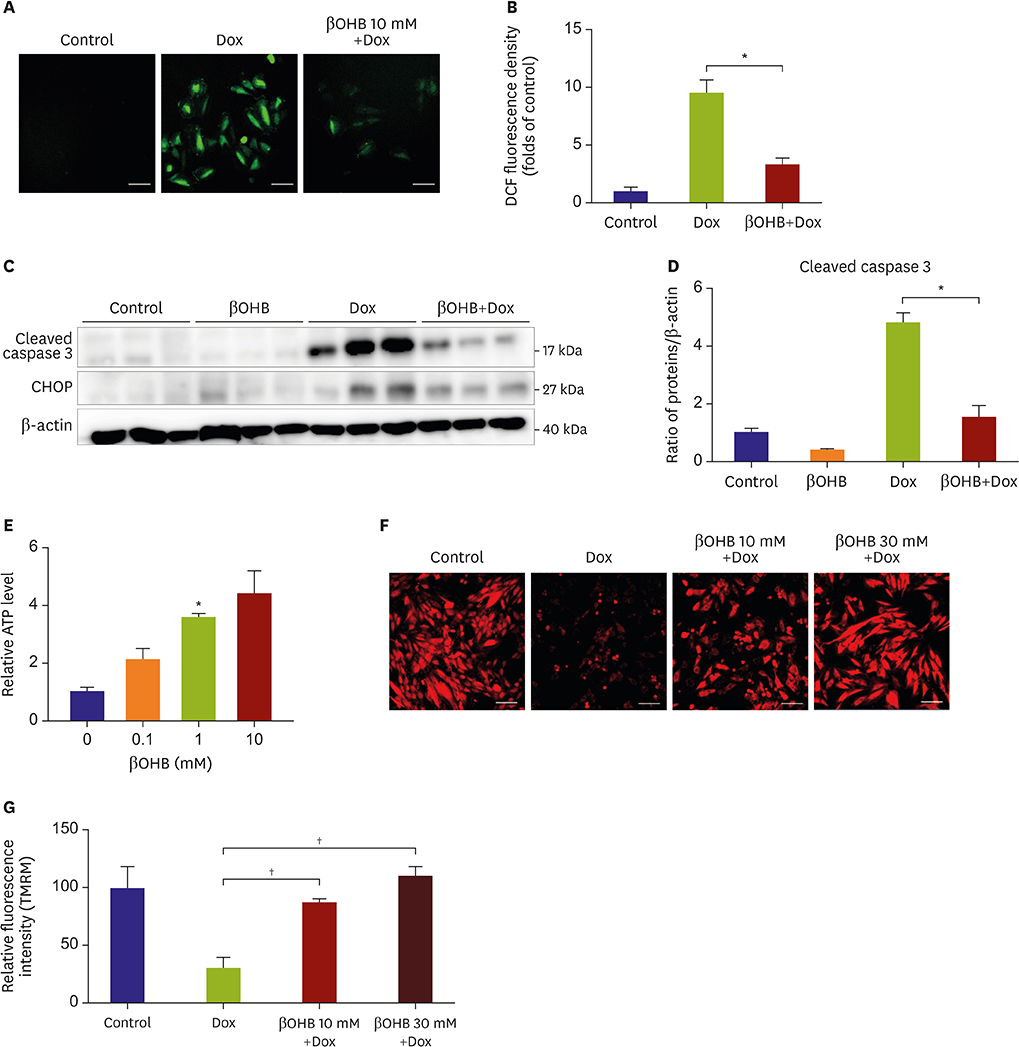
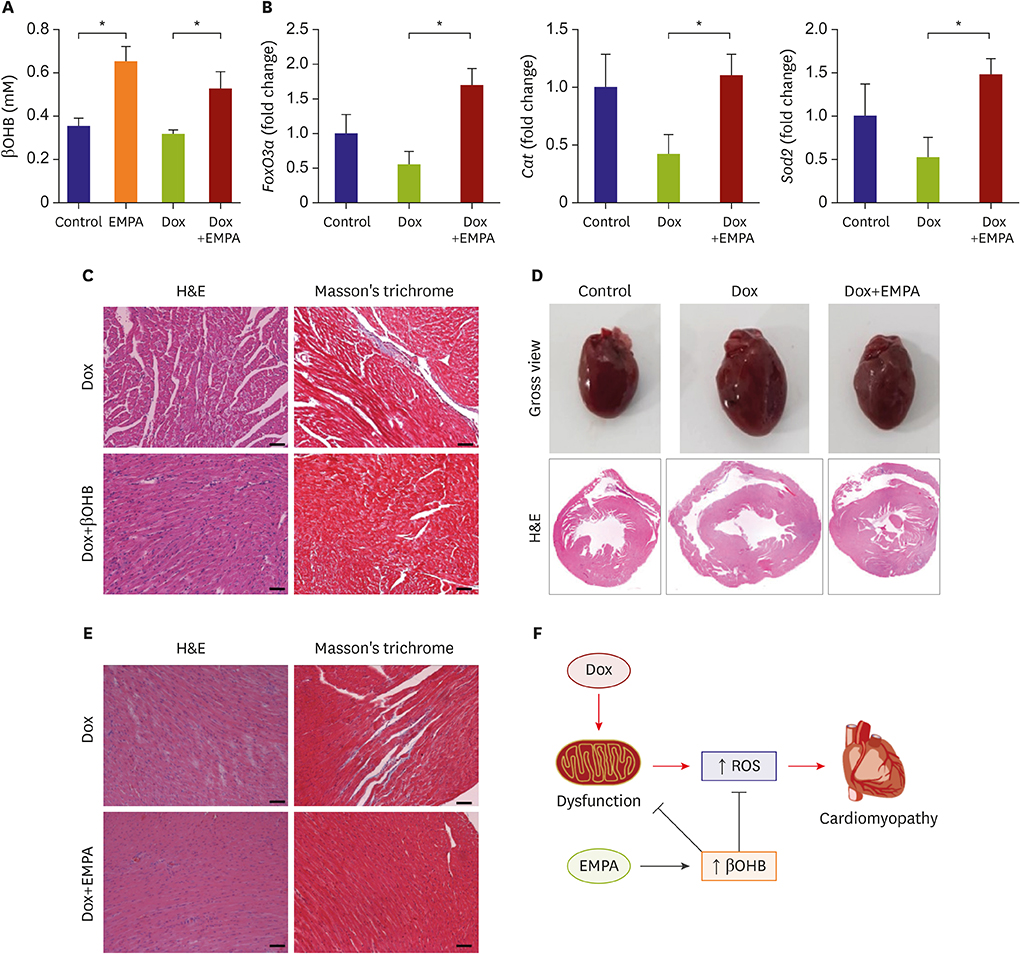
Mice fed NCD with EMPA showed improved heart function and reduced fibrosis. In vitro studies showed similar results. Phloridzin, a non-specific SGLT inhibitor, did not show any protective effect against Dox toxicity in H9C2 cells. SGLT2 inhibitor can cause increase in blood ketone levels. Beta hydroxybutyrate (βOHB), which is well known ketone body associated with SGLT2 inhibitor, showed a protective effect against Dox in H9C2 cells and in Dox-treated mice. These results suggest elevating βOHB might be a convincing mechanism for the protective effects of SGLT2 inhibitor.
CONCLUSIONS
SGLT2 inhibitors have a protective effect in Dox-induced HF in mice. This implied that SGLT2 inhibitor therapy could be a good treatment strategy even in HF patients without diabetes.
MeSH Terms
Figure
Cited by 2 articles
-
Heart Failure with Preserved Ejection Fraction: the Major Unmet Need in Cardiology
Chi Young Shim
Korean Circ J. 2020;50(12):1051-1061. doi: 10.4070/kcj.2020.0338.Pharmacologic Activation of Angiotensin-Converting Enzyme II Alleviates Diabetic Cardiomyopathy in
db/db Mice by Reducing Reactive Oxidative Stress
Donghyun Kim, Wooju Jeong, Yumin Kim, Jibeom Lee, Sung Woo Cho, Chang-Myung Oh, Raekil Park
Diabetes Metab J. 2023;47(4):487-499. doi: 10.4093/dmj.2022.0125.
Reference
-
1. Berliner D, Bauersachs J. Current drug therapy in chronic heart failure: the new guidelines of the European Society of Cardiology (ESC). Korean Circ J. 2017; 47:543–554.2. Abdul-Ghani M, Del Prato S, Chilton R, DeFronzo RA. SGLT2 inhibitors and cardiovascular risk: lessons learned from the EMPA-REG OUTCOME study. Diabetes Care. 2016; 39:717–725.
Article3. Mudaliar S, Polidori D, Zambrowicz B, Henry RR. Sodium-glucose cotransporter inhibitors: effects on renal and intestinal glucose transport: from bench to bedside. Diabetes Care. 2015; 38:2344–2353.4. Ferrannini E, Baldi S, Frascerra S, et al. Shift to fatty substrate utilization in response to sodium–glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes. 2016; 65:1190–1195.
Article5. Grabacka M, Pierzchalska M, Dean M, Reiss K. Regulation of ketone body metabolism and the role of PPARα. Int J Mol Sci. 2016; 17:E2093.6. Vettor R, Inzucchi SE, Fioretto P. The cardiovascular benefits of empagliflozin: SGLT2-dependent and -independent effects. Diabetologia. 2017; 60:395–398.
Article7. Kong G, Huang Z, Ji W, et al. The ketone metabolite β-hydroxybutyrate attenuates oxidative stress in spinal cord injury by suppression of class I histone deacetylases. J Neurotrauma. 2017; 34:2645–2655.8. Napolitano A, Miller S, Murgatroyd PR, et al. Exploring glycosuria as a mechanism for weight and fat mass reduction. A pilot study with remogliflozin etabonate and sergliflozin etabonate in healthy obese subjects. J Clin Transl Endocrinol. 2013; 1:e3–8.
Article9. Thapa S, Trivedi N, Omer A. Elevated serum beta-hydroxybutyrate levels (B-hb) in patients with type 2 diabetes mellitus using sodium-glucose cotransporter 2 (SGLT-2) inhibitor. Novel treatment for diabetes-focusing on GLP-1 and SGLT2 (posters). In : The 98th Annual Meeting of the Endocrine Society; 2016 Apr 1–4; Fri, USA. Boston (MA): Endocrine Society;2016.10. Breckenridge R. Heart failure and mouse models. Dis Model Mech. 2010; 3:138–143.
Article11. Jin Z, Zhang J, Zhi H, et al. Beneficial effects of tadalafil on left ventricular dysfunction in doxorubicin-induced cardiomyopathy. J Cardiol. 2013; 62:110–116.12. Gao S, Ho D, Vatner DE, Vatner SF. Echocardiography in mice. Curr Protoc Mouse Biol. 2011; 1:71–83.
Article13. Lee BS, Kim SH, Jin T, et al. Protective effect of survivin in doxorubicin-induced cell death in h9c2 cardiac myocytes. Korean Circ J. 2013; 43:400–407.14. Joshi DC, Bakowska JC. Determination of mitochondrial membrane potential and reactive oxygen species in live rat cortical neurons. J Vis Exp. 2011; 2704.
Article15. Mouli S, Nanayakkara G, AlAlasmari A, et al. The role of frataxin in doxorubicin-mediated cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2015; 309:H844–59.16. Chen J, Williams S, Ho S, et al. Quantitative PCR tissue expression profiling of the human SGLT2 gene and related family members. Diabetes Ther. 2010; 1:57–92.
Article17. Lopaschuk GD, Verma S. Empagliflozin's fuel hypothesis: not so soon. Cell Metab. 2016; 24:200–202.18. Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care. 2015; 38:1638–1642.
Article19. Guh JY, Chuang TD, Chen HC, et al. β-hydroxybutyrate-induced growth inhibition and collagen production in HK-2 cells are dependent on TGF-β and Smad3. Kidney Int. 2003; 64:2041–2051.20. Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, Moens AL. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol. 2012; 52:1213–1225.
Article21. Mitry MA, Edwards JG. Doxorubicin induced heart failure: phenotype and molecular mechanisms. Int J Cardiol Heart Vasc. 2016; 10:17–24.22. Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015; 21:263–269.
Article23. Liu X, Wang X, Zhang X, Xie Y, Chen R, Chen H. C57BL/6 mice are more appropriate than BALB/C mice in inducing dilated cardiomyopathy with short-term doxorubicin treatment. Acta Cardiol Sin. 2012; 28:236–240.24. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015; 373:2117–2128.
Article25. Shimazu T, Hirschey MD, Newman J, et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013; 339:211–214.26. Sampson M, Lathen DR, Dallon BW, et al. β-Hydroxybutyrate improves β-cell mitochondrial function and survival. J Insul Resist. 2017; 2:8.
Article27. Lim S, Chesser AS, Grima JC, et al. D-β-hydroxybutyrate is protective in mouse models of Huntington's disease. PLoS One. 2011; 6:e24620.28. Aubert G, Martin OJ, Horton JL, et al. The failing heart relies on ketone bodies as a fuel. Circulation. 2016; 133:698–705.
Article29. Yu Y, Yu Y, Zhang Y, Zhang Z, An W, Zhao X. Treatment with D-β-hydroxybutyrate protects heart from ischemia/reperfusion injury in mice. Eur J Pharmacol. 2018; 829:121–128.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Potential Cardioprotective Mechanism of Sodium-Glucose Cotransporter 2 Inhibitors
- SGLT2 Inhibitors: Emerging Drugs in Heart Failure
- Fasting is not always good: perioperative fasting leads to pronounced ketone body production in patients treated with SGLT2 inhibitors: a case report
- SGLT2 Inhibitors and Diabetes: Where Does It Come from and Where Does It Go?
- Acute Kidney Injury after Administering Dapagliflozin to a Diabetic Patient with Acute Cerebral Infarction