Pediatr Gastroenterol Hepatol Nutr.
2019 Nov;22(6):518-526. 10.5223/pghn.2019.22.6.518.
Cutaneous Patches to Monitor Myoelectric Activity of the Gastrointestinal Tract in Postoperative Pediatric Patients
- Affiliations
-
- 1Department of Surgery, Division of Pediatric Surgery, Stanford University School of Medicine, Stanford, CA, USA. jkwall@stanford.edu
- 2G-Tech Medical, Fogarty Institute for Innovation, Mountain View, CA, USA.
- KMID: 2462091
- DOI: http://doi.org/10.5223/pghn.2019.22.6.518
Abstract
- PURPOSE
Limited means exist to assess gastrointestinal activity in pediatric patients postoperatively. Recently, myoelectric gastrointestinal activity recorded by cutaneous patches has been shown in adult patients to be predictive of clinical return of gastrointestinal function postoperatively. The aim of this case series is to demonstrate the feasibility of this system in pediatric patients and to correlate myoelectric signals with return of bowel function clinically.
METHODS
Pediatric patients undergoing abdominal surgery were recruited to have wireless patches placed on the abdomen within two hours postoperatively. Myoelectric data were transmitted wirelessly to a mobile device with a user-interface and forwarded to a cloud server where processing algorithms identified episodes of motor activity, quantified their parameters and nominally assigned them to specific gastrointestinal organs based on their frequencies.
RESULTS
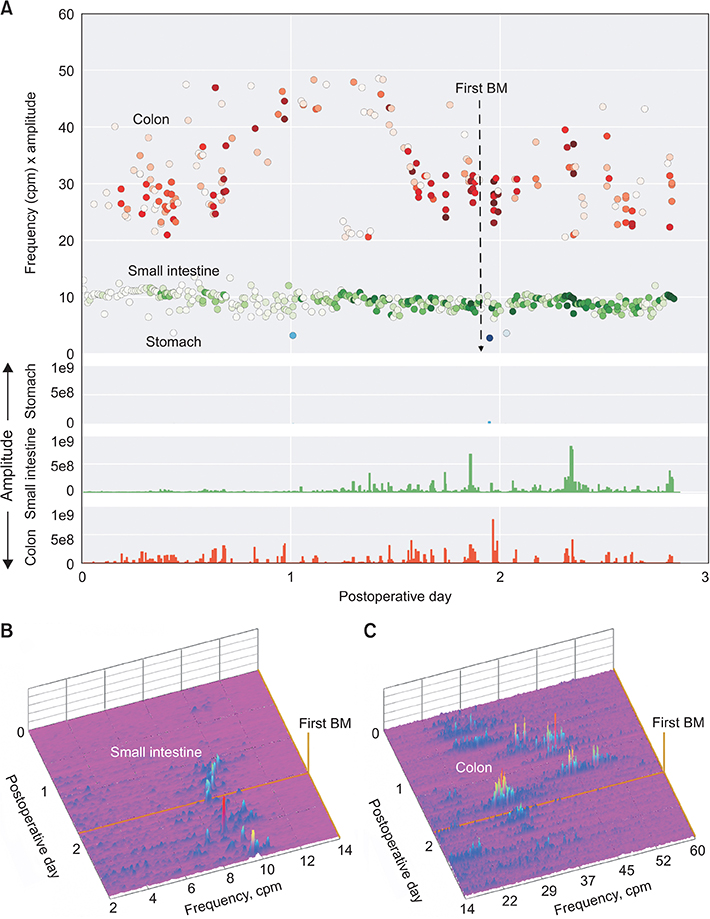
Three patients (ages 5 months, 4 year, 16 year) were recruited for this study. Multiple patches were placed on the older subjects, while the youngest had a single patch due to space limitations. Rhythmic signals of the stomach, small intestine, and colon could be identified in all three subjects. Patients showed gradual increase in myoelectric intestinal and colonic activity leading up to the first recorded bowel movement.
CONCLUSION
Measuring myoelectric intestinal activity continuously using a wireless patch system is feasible in a wide age range of pediatric patients. The increase in activity over time correlated well with the patients' return of bowel function. More studies are planned to determine if this technology can predict return of bowel function or differentiate between physiologic ileus and pathologic conditions.
MeSH Terms
Figure
Reference
-
1. Holte K, Kehlet H. Postoperative ileus: a preventable event. Br J Surg. 2000; 87:1480–1493.
Article2. Wilson JP. Postoperative motility of the large intestine in man. Gut. 1975; 16:689–692.
Article3. Luckey A, Livingston E, Taché Y. Mechanisms and treatment of postoperative ileus. Arch Surg. 2003; 138:206–214.
Article4. Mattei P, Rombeau JL. Review of the pathophysiology and management of postoperative ileus. World J Surg. 2006; 30:1382–1391.
Article5. Wolthuis AM, Bislenghi G, Fieuws S, de Buck van Overstraeten A, Boeckxstaens G, D'Hoore A. Incidence of prolonged postoperative ileus after colorectal surgery: a systematic review and meta-analysis. Colorectal Dis. 2016; 18:O1–O9.
Article6. Kulaylat AN, Rocourt DV, Tsai AY, Martin KL, Engbrecht BW, Santos MC, et al. Understanding readmissions in children undergoing surgery: a pediatric NSQIP analysis. J Pediatr Surg. 2018; 53:1280–1287.
Article7. Condon RE, Cowles VE, Ferraz AA, Carilli S, Carlson ME, Ludwig K, et al. Human colonic smooth muscle electrical activity during and after recovery from postoperative ileus. Am J Physiol. 1995; 269(3 Pt 1):G408–G417.
Article8. Waldhausen JH, Shaffrey ME, Skenderis BS 2nd, Jones RS, Schirmer BD. Gastrointestinal myoelectric and clinical patterns of recovery after laparotomy. Ann Surg. 1990; 211:777–784. discussion 785.
Article9. Dua MM, Navalgund A, Axelrod S, Axelrod L, Worth PJ, Norton JA, et al. Monitoring gastric myoelectric activity after pancreaticoduodenectomy for diet “readiness”. Am J Physiol Gastrointest Liver Physiol. 2018; 315:G743–G751.
Article10. Navalgund A, Axelrod S, Axelrod L, Singhal S, Tran K, Legha P, et al. Colon myoelectric activity measured after open abdominal surgery with a noninvasive wireless patch system predicts time to first flatus. J Gastrointest Surg. 2019; 23:982–989.
Article11. Axelrod LA, Navalgund AR, Axelrod S, Triadafilopoulos G. Mo1592 - frequency spectra from 72 hour external myoelectric measurements of the gut demonstrate strong reproducibility as well as sensitivity to drug-induced stimulation. Gastroenterol. 2018; 154:S-763.
Article12. Axelrod S, Navalgund AR, Axelrod LA, Triadafilopoulos G. Mo1591 - a new motility tool: high concordance between internal smartpill pressure recordings and myoelectric events measured by external wireless g-tech patches. Gastroenterol. 2018; 154:S-763.
Article13. Barletta JF, Senagore AJ. Reducing the burden of postoperative ileus: evaluating and implementing an evidence-based strategy. World J Surg. 2014; 38:1966–1977.
Article14. Grass F, Slieker J, Jurt J, Kummer A, Solà J, Hahnloser D, et al. Postoperative ileus in an enhanced recovery pathway-a retrospective cohort study. Int J Colorectal Dis. 2017; 32:675–681.
Article15. Shinnick JK, Short HL, Heiss KF, Santore MT, Blakely ML, Raval MV. Enhancing recovery in pediatric surgery: a review of the literature. J Surg Res. 2016; 202:165–176.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antimicrobial therapy for pediatric gastrointestinal tract infections
- Stem Cell in Pediatric Gastrointestinal Tract Disease: Hirschsprung Disease
- Cutaneous Sarcoidosis Presenting as Multiple Erythematous Macules and Patches
- Recent Achievements in Stem Cell Therapy for Pediatric Gastrointestinal Tract Disease
- The Role of Capsaicin in Spontaneous Pacemaking Activity in Gastrointestinal Tract