Korean J Physiol Pharmacol.
2019 Nov;23(6):467-474. 10.4196/kjpp.2019.23.6.467.
Influence of clozapine on neurodevelopmental protein expression and behavioral patterns in animal model of psychiatric disorder induced by low-level of lead
- Affiliations
-
- 1Department of Clinical Pharmacology, College of Medicine, Soonchunhyang University, Cheonan 31151, Korea. hak3962@sch.ac.kr
- 2Department of Bio-Environmental Chemistry, College of Agriculture and Life Sciences, Chungnam National University, Daejeon 34134, Korea.
- KMID: 2461039
- DOI: http://doi.org/10.4196/kjpp.2019.23.6.467
Abstract
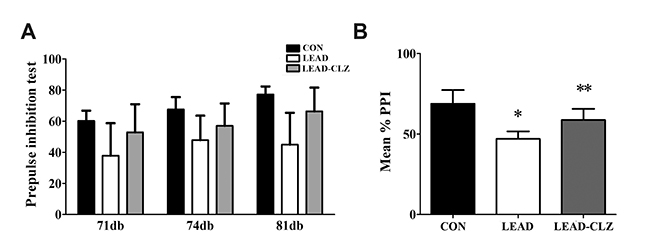
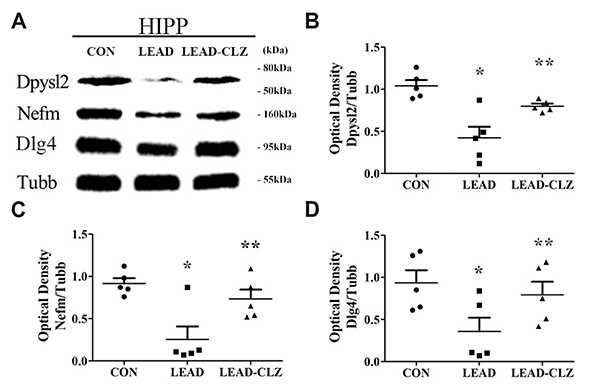
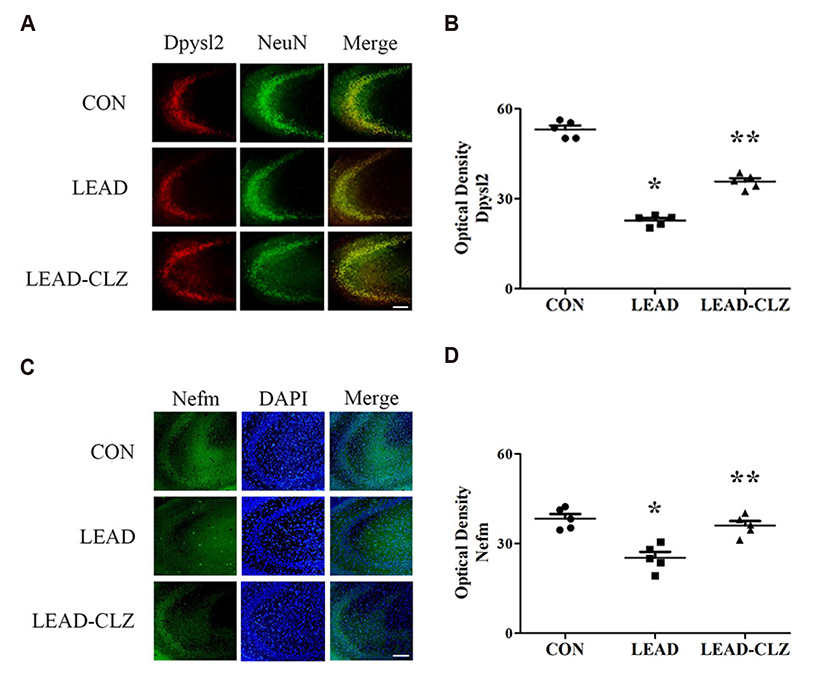
- Exposure to lead during pregnancy is a risk factor for the development of psychiatric disorders in the offspring. In this study, we investigated whether exposure to low levels of lead acetate (0.2%) in drinking water during pregnancy and lactation causes behavioral impairment and affects the expression of proteins associated with neurodevelopment. Lead exposure altered several parameters in rat offspring compared with those unexposed in open-field, social interaction, and pre-pulse inhibition tests. These parameters were restored to normal levels after clozapine treatment. Western blot and immunohistochemical analyses of the hippocampus revealed that several neurodevelopmental proteins were downregulated in lead-exposed rats. The expression was normalized after clozapine treatment (5 mg/kg/day, postnatal day 35-56). These findings demonstrate that downregulation of several proteins in lead-exposed rats affected subsequent behavioral changes. Our results suggest that lead exposure in early life may induce psychiatric disorders and treatment with antipsychotics such as clozapine may reduce their incidence.
MeSH Terms
Figure
Reference
-
1. Liu JT, Chen BY, Zhang JQ, Kuang F, Chen LW. Lead exposure induced microgliosis and astrogliosis in hippocampus of young mice potentially by triggering TLR4-MyD88-NFκB signaling cascades. Toxicol Lett. 2015; 239:97–107.
Article2. Guilarte TR, Opler M, Pletnikov M. Is lead exposure in early life an environmental risk factor for Schizophrenia? neurobiological connections and testable hypotheses. Neurotoxicology. 2012; 33:560–574.
Article3. Moreira EG, Vassilieff I, Vassilieff VS. Developmental lead exposure: behavioral alterations in the short and long term. Neurotoxicol Teratol. 2001; 23:489–495.
Article4. Needleman HL, Gunnoe C, Leviton A, Reed R, Peresie H, Maher C, Barrett P. Deficits in psychologic and classroom performance of children with elevated dentine lead levels. N Engl J Med. 1979; 300:689–695.
Article5. Dietrich KN, Berger OG, Bhattacharya A. Symptomatic lead poisoning in infancy: a prospective case analysis. J Pediatr. 2000; 137:568–571.
Article6. Olympio KP, Gonçalves C, Risso Günther WM, Henriques Bechara EJ. Neurotoxicity and aggressiveness triggered by low-level lead in children: a review. Rev Panam Salud Publica. 2009; 26:266–275.
Article7. Hu H, Téllez-Rojo MM, Bellinger D, Smith D, Ettinger AS, Lamadrid-Figueroa H, Schwartz J, Schnaas L, Mercado-García A, Hernández-Avila M. Fetal lead exposure at each stage of pregnancy as a predictor of infant mental development. Environ Health Perspect. 2006; 114:1730–1735.
Article8. Budtz-Jørgensen E, Bellinger D, Lanphear B, Grandjean P. International Pooled Lead Study Investigators. An international pooled analysis for obtaining a benchmark dose for environmental lead exposure in children. Risk Anal. 2013; 33:450–461.
Article9. Boksa P. Animal models of obstetric complications in relation to schizophrenia. Brain Res Brain Res Rev. 2004; 45:1–17.
Article10. Kvajo M, McKellar H, Gogos JA. Avoiding mouse traps in schizophrenia genetics: lessons and promises from current and emerging mouse models. Neuroscience. 2012; 211:136–164.
Article11. White LD, Cory-Slechta DA, Gilbert ME, Tiffany-Castiglioni E, Zawia NH, Virgolini M, Rossi-George A, Lasley SM, Qian YC, Basha MR. New and evolving concepts in the neurotoxicology of lead. Toxicol Appl Pharmacol. 2007; 225:1–27.
Article12. Lasley SM, Gilbert ME. Glutamatergic components underlying leadinduced impairments in hippocampal synaptic plasticity. Neurotoxicology. 2000; 21:1057–1068.13. Brini M, Calì T, Ottolini D, Carafoli E. Neuronal calcium signaling: function and dysfunction. Cell Mol Life Sci. 2014; 71:2787–2814.
Article14. Rao Barkur R, Bairy LK. Evaluation of passive avoidance learning and spatial memory in rats exposed to low levels of lead during specific periods of early brain development. Int J Occup Med Environ Health. 2015; 28:533–544.
Article15. Lanphear BP, Dietrich K, Auinger P, Cox C. Cognitive deficits associated with blood lead concentrations <10 microg/dL in US children and adolescents. Public Health Rep. 2000; 115:521–529.16. Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978; 15:339–343.
Article17. Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry. 1992; 49:206–215.
Article18. Geyer MA. The family of sensorimotor gating disorders: comorbidities or diagnostic overlaps. Neurotox Res. 2006; 10:211–220.
Article19. Pouzet B, Andersen MP, Hogg S. Effects of acute treatment with antidepressant drugs on sensorimotor gating deficits in rats. Psychopharmacology (Berl). 2005; 178:9–16.
Article20. Le Pen G, Moreau JL. Disruption of prepulse inhibition of startle reflex in a neurodevelopmental model of schizophrenia: reversal by clozapine, olanzapine and risperidone but not by haloperidol. Neuropsychopharmacology. 2002; 27:1–11.
Article21. Khan AH, Zaidi S. Clozapine: improvement of negative symptoms of schizophrenia. Cureus. 2017; 9:e1973.
Article22. Won HS, Kim YO, Lee HY, Im JY, Lee SH, Cho IH, Lee SW, Park CG, Kim HK, Kwon JT, Kim HJ. Effect of valeriana fauriei extract on the neurodevelopmental proteins expression and behavioral patterns in maternal immune activation animal model. Korean J Med Crop Sci. 2016; 24:341–350.
Article23. O'Shea PJ, Harvey CB, Suzuki H, Kaneshige M, Kaneshige K, Cheng SY, Williams GR. A thyrotoxic skeletal phenotype of advanced bone formation in mice with resistance to thyroid hormone. Mol Endocrinol. 2003; 17:1410–1424.24. Bradshaw NJ, Porteous DJ. DISC1-binding proteins in neural development, signalling and schizophrenia. Neuropharmacology. 2012; 62:1230–1241.
Article25. Taya S, Shinoda T, Tsuboi D, Asaki J, Nagai K, Hikita T, Kuroda S, Kuroda K, Shimizu M, Hirotsune S, Iwamatsu A, Kaibuchi K. DISC1 Regulates the Transport of the NUDEL/LIS1/14-3-3epsilon Complex through Kinesin-1. J Neurosci. 2007; 27:15–26.26. Soares DC, Carlyle BC, Bradshaw NJ, Porteous DJ. DISC1: Structure, Function, and Therapeutic Potential for Major Mental Illness. ACS Chem Neurosci. 2011; 2:609–632.
Article27. Shinoda T, Taya S, Tsuboi D, Hikita T, Matsuzawa R, Kuroda S, Iwamatsu A, bKaibuchi K. DISC1 regulates neurotrophin-induced axon elongation via interaction with Grb2. J Neurosci. 2007; 27:4–14.
Article28. Institute of Laboratory Animal Resources (Estats Units d'Amèrica). Guide for the care and use of laboratory animals. Washington: National Academy Press;1996. p. xii–125.29. Schroeder M, Sultany T, Weller A. Prenatal stress effects on emotion regulation differ by genotype and sex in prepubertal rats. Dev Psychobiol. 2013; 55:176–192.
Article30. Lee H, Won H, Im J, Kim YO, Lee S, Cho IH, Kim HK, Kwon JT, Kim HJ. Effect of Valeriana fauriei extract on the offspring of adult rats exposed to prenatal stress. Int J Mol Med. 2016; 38:251–258.
Article31. Jung HY, Chung TH, Hwang IK. Dendropanax morbifera Léveille extract ameliorates memory impairments and inflammatory responses in the hippocampus of streptozotocin-induced type 1 diabetic rats. Mol Cell Toxicol. 2016; 12:429–436.
Article32. Altmann L, Weinsberg F, Sveinsson K, Lilienthal H, Wiegand H, Winneke G. Impairment of long-term potentiation and learning following chronic lead exposure. Toxicol Lett. 1993; 66:105–112.
Article33. Chen HH, Ma T, Paul IA, Spencer JL, Ho IK. Developmental lead exposure and two-way active avoidance training alter the distribution of protein kinase C activity in the rat hippocampus. Neurochem Res. 1997; 22:1119–1125.34. Ma T, Chen HH, Ho IK. Effects of chronic lead (Pb) exposure on neurobehavioral function and dopaminergic neurotransmitter receptors in rats. Toxicol Lett. 1999; 105:111–121.
Article35. Commissaris RL, Tavakoli-Nezhad M, Barron AJ, Pitts DK. Effects of chronic low-level oral lead exposure on prepulse inhibition of acoustic startle in the rat. Neurotoxicol Teratol. 2000; 22:55–60.
Article36. Vollenweider FX, Barro M, Csomor PA, Feldon J. Clozapine enhances prepulse inhibition in healthy humans with low but not with high prepulse inhibition levels. Biol Psychiatry. 2006; 60:597–603.
Article37. Kumari V, Soni W, Sharma T. Normalization of information processing deficits in schizophrenia with clozapine. Am J Psychiatry. 1999; 156:1046–1051.38. Xu J, Yan HC, Yang B, Tong LS, Zou YX, Tian Y. Effects of lead exposure on hippocampal metabotropic glutamate receptor subtype 3 and 7 in developmental rats. J Negat Results Biomed. 2009; 8:5.
Article39. Neal AP, Worley PF, Guilarte TR. Lead exposure during synaptogenesis alters NMDA receptor targeting via NMDA receptor inhibition. Neurotoxicology. 2011; 32:281–289.
Article40. Hossain S, Bhowmick S, Jahan S, Rozario L, Sarkar M, Islam S, Basunia MA, Rahman A, Choudhury BK, Shahjalal H. Maternal lead exposure decreases the levels of brain development and cognitionrelated proteins with concomitant upsurges of oxidative stress, inflammatory response and apoptosis in the offspring rats. Neurotoxicology. 2016; 56:150–158.
Article41. Yokoyama K, Araki S. Assessment of slow axonal transport in leadexposed rats. Environ Res. 1992; 59:440–446.
Article42. Joo J, Lee S, Nah SS, Kim YO, Kim DS, Shim SH, Hwangbo Y, Kim HK, Kwon JT, Kim JW, Song HY, Kim HJ. Lasp1 is down-regulated in NMDA receptor antagonist-treated mice and implicated in human schizophrenia susceptibility. J Psychiatr Res. 2013; 47:105–112.
Article43. Calabrese F, Guidotti G, Molteni R, Racagni G, Mancini M, Riva MA. Stress-induced changes of hippocampal NMDA receptors: modulation by duloxetine treatment. PLoS One. 2012; 7:e37916.
Article44. Rubio MD, Drummond JB, Meador-Woodruff JH. Glutamate receptor abnormalities in schizophrenia: implications for innovative treatments. Biomol Ther (Seoul). 2012; 20:1–18.
Article45. Lee H, Joo J, Nah SS, Kim JW, Kim HK, Kwon JT, Lee HY, Kim YO, Kim HJ. Changes in Dpysl2 expression are associated with prenatally stressed rat offspring and susceptibility to schizophrenia in humans. Int J Mol Med. 2015; 35:1574–1586.
Article46. Sur C, Mallorga PJ, Wittmann M, Jacobson MA, Pascarella D, Williams JB, Brandish PE, Pettibone DJ, Scolnick EM, Conn PJ. Ndesmethylclozapine, an allosteric agonist at muscarinic 1 receptor, potentiates N-methyl-D-aspartate receptor activity. Proc Natl Acad Sci U S A. 2003; 100:13674–13679.
Article47. von Wilmsdorff M, Manthey F, Bouvier ML, Staehlin O, Falkai P, Meisenzahl-Lechner E, Schmitt A, Gebicke-Haerter PJ. Effects of haloperidol and clozapine on synapse-related gene expression in specific brain regions of male rats. Eur Arch Psychiatry Clin Neurosci. 2018; 268:555–563.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Clozapine Induced Acute Renal Failure
- Three Cases of Reversible Agranulocytosis after Treatment with Lamotrigine
- Exposure to Lead and Attentional and Behavioral Problems in Attention Deficit Hyperactivity Disorder
- Clonidine Treatment of Clozapine-Induced Hypersalivation
- A Case of Clozapine Treatment of Parkinsonism with Delusional Disorder