Korean J Gastroenterol.
2019 Oct;74(4):227-231. 10.4166/kjg.2019.74.4.227.
Synchronous Gastrointestinal Stromal Tumor and Ampullary Neuroendocrine Tumor in Association with Neurofibromatosis Type 1: A Report of Three Cases
- Affiliations
-
- 1Department of Surgery, Chonnam National University Hospital, Gwangju, Korea.
- 2Department of Surgery, Chonnam National University Hwasun Hospital, Chonnam National University Medical School, Hwasun, Korea. ckcho@jnu.ac.kr
- KMID: 2460988
- DOI: http://doi.org/10.4166/kjg.2019.74.4.227
Abstract
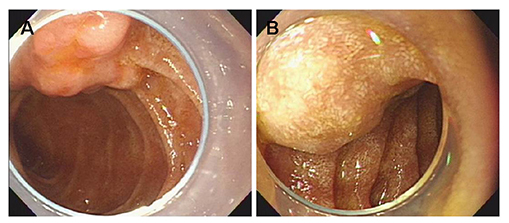
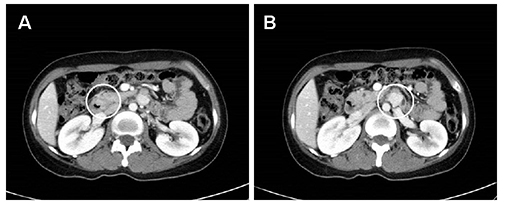
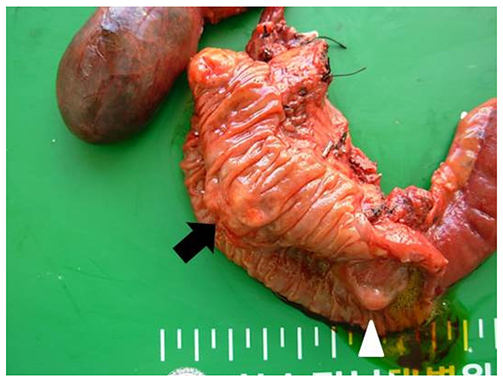
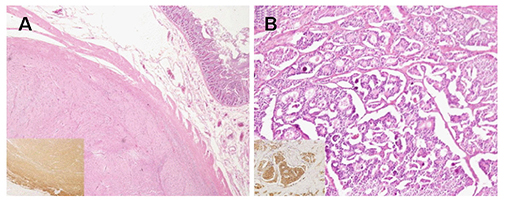
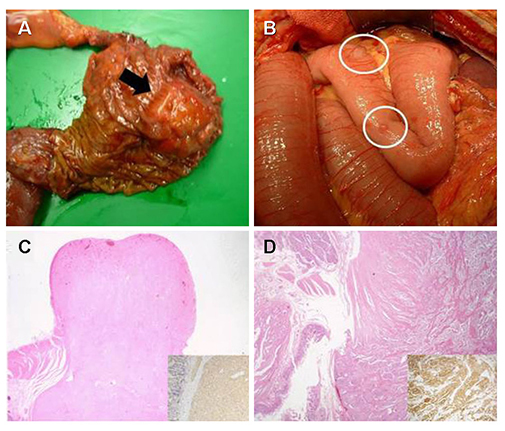
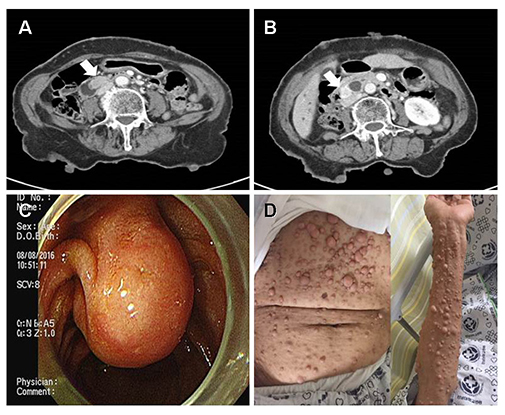
- Neurofibromatosis type 1 (NF1) is an autosomal dominant hereditary disorder. The pathogenesis of NF1 is suggested to be an alteration of the NF-1 gene, which normally functions as a tumor suppressor. A mutation of NF-1 causes the development of viable tumors in various sites. On the other hand, the synchronous manifestation of a gastrointestinal stromal tumor (GIST) and neuroendocrine tumor (NET) in the background of NF1 is extremely rare. This paper reports three cases treated with surgical intervention along with the long-term follow-up results. Three patients showed synchronous ampullary NET and GIST in association with NF1 supported by postoperative histopathologic analysis. Surgical treatments, such as pancreatoduodenectomy and local excision were applied. No recurrence occurred during the postoperative follow-up period of 10, 9, and 2.7 years. Synchronous GIST and NET in the background of NF1 is extremely rare, but the possible coexistence of other tumors in NF1 patients is relatively higher than that in the general population. Furthermore, both NETs and GISTs occurring in NF1 patients tend to be smaller in size compared to that in the general population. Therefore, when NF1 patients present with vague abdominal discomfort, close attention must be paid to identifying the coexistence of other neoplasms.
Keyword
MeSH Terms
Figure
Reference
-
1. Ferner RE. Neurofibromatosis 1 and neurofibromatosis 2: a twenty first century perspective. Lancet Neurol. 2007; 6:340–351.
Article2. Gottfried ON, Viskochil DH, Couldwell WT. Neurofibromatosis type 1 and tumorigenesis: molecular mechanisms and therapeutic implications. Neurosurg Focus. 2010; 28:E8.
Article3. Agaimy A, Vassos N, Croner RS. Gastrointestinal manifestations of neurofibromatosis type 1 (Recklinghausen's disease): clinicopathological spectrum with pathogenetic considerations. Int J Clin Exp Pathol. 2012; 5:852–862.4. Levy AD, Patel N, Dow N, Abbott RM, Miettinen M, Sobin LH. Abdominal neoplasms in patients with neurofibromatosis type 1: radiologic-pathologic correlation. Radiographics. 2005; 25:455–480.
Article5. Fuller CE, Williams GT. Gastrointestinal manifestations of type 1 neurofibromatosis (von Recklinghausen's disease). Histopathology. 1991; 19:1–11.
Article6. Ferner RE, Huson SM, Thomas N, et al. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J Med Genet. 2007; 44:81–88.
Article7. Salvi PF, Lorenzon L, Caterino S, Antolino L, Antonelli MS, Balducci G. Gastrointestinal stromal tumors associated with neurofibromatosis 1: a single centre experience and systematic review of the literature including 252 cases. Int J Surg Oncol. 2013; 2013:398570.
Article8. Relles D, Baek J, Witkiewicz A, Yeo CJ. Periampullary and duodenal neoplasms in neurofibromatosis type 1: two cases and an updated 20-year review of the literature yielding 76 cases. J Gastrointest Surg. 2010; 14:1052–1061.
Article9. Hirbe AC, Gutmann DH. Neurofibromatosis type 1: a multidisciplinary approach to care. Lancet Neurol. 2014; 13:834–843.
Article10. Garrouche N, Ben Abdallah A, Arifa N, et al. Spectrum of gastrointestinal lesions of neurofibromatosis type 1: a pictorial review. Insights Imaging. 2018; 9:661–671.
Article11. Nikfarjam M, McLean C, Muralidharan V, Christophi C. Neuroendocrine tumours of the ampulla of Vater. ANZ J Surg. 2002; 72:531–533.
Article12. Watanabe Y, Yamada D, Ogawa Y, et al. Long-term survival of a patient with neuroendocrine carcinoma of the ampulla of Vater with lymph node micrometastasis. Am Surg. 2013; 79:E199–E201.
Article13. Hwang S, Lee SG, Lee YJ, et al. Radical surgical resection for carcinoid tumors of the ampulla. J Gastrointest Surg. 2008; 12:713–717.
Article14. Fjällskog ML, Granberg DP, Welin SL, et al. Treatment with cisplatin and etoposide in patients with neuroendocrine tumors. Cancer. 2001; 92:1101–1107.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Two Cases of Neuroendocrine Carcinoma and GIST in a Patient with Neurofibromatosis Type 1
- Solitary Malignant Gastrointestinal Stromal Tumor Associated with a Neurofibromatosis Type I
- Gastrointestinal stromal tumor with KIT mutation in neurofibromatosis type 1
- Multiple Small Intestinal Stromal Tumors Associated with Neurofibromatosis-1
- Synchronous Occurrence of a Gastric Adenocarcinoma and a GIST (Gastrointestinal Stromal Tumor): A Case Report