Cancer Res Treat.
2019 Oct;51(4):1437-1448. 10.4143/crt.2018.611.
Impact of 21-Gene Recurrence Score on Chemotherapy Decision in Invasive Ductal Carcinoma of Breast with Nodal Micrometastases
- Affiliations
-
- 1Department of Breast Surgery, Zhuhai Maternity and Child Health Hospital, Zhuhai, China.
- 2Department of Radiation Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, Guangzhou, China. hezhy@sysucc.org.cn
- 3Department of Radiation Oncology, Cancer Hospital, The First Affiliated Hospital of Xiamen University, Teaching Hospital of Fujian Medical University, Xiamen, China. unowu12345@hotmail.com
- KMID: 2460593
- DOI: http://doi.org/10.4143/crt.2018.611
Abstract
- PURPOSE
The purpose of this study was to investigate the effect of 21-gene recurrence score (RS) on predicting prognosis and chemotherapy decision in node micrometastases (N1mi) breast invasive ductal carcinoma (IDC).
MATERIALS AND METHODS
Patients with stage T1-2N1mi and estrogen receptor-positive IDC diagnosed between 2004 and 2015 were included. The associations of 21-gene RS with breast cancer-specific survival (BCSS), chemotherapy decision, and benefit of chemotherapy were analyzed.
RESULTS
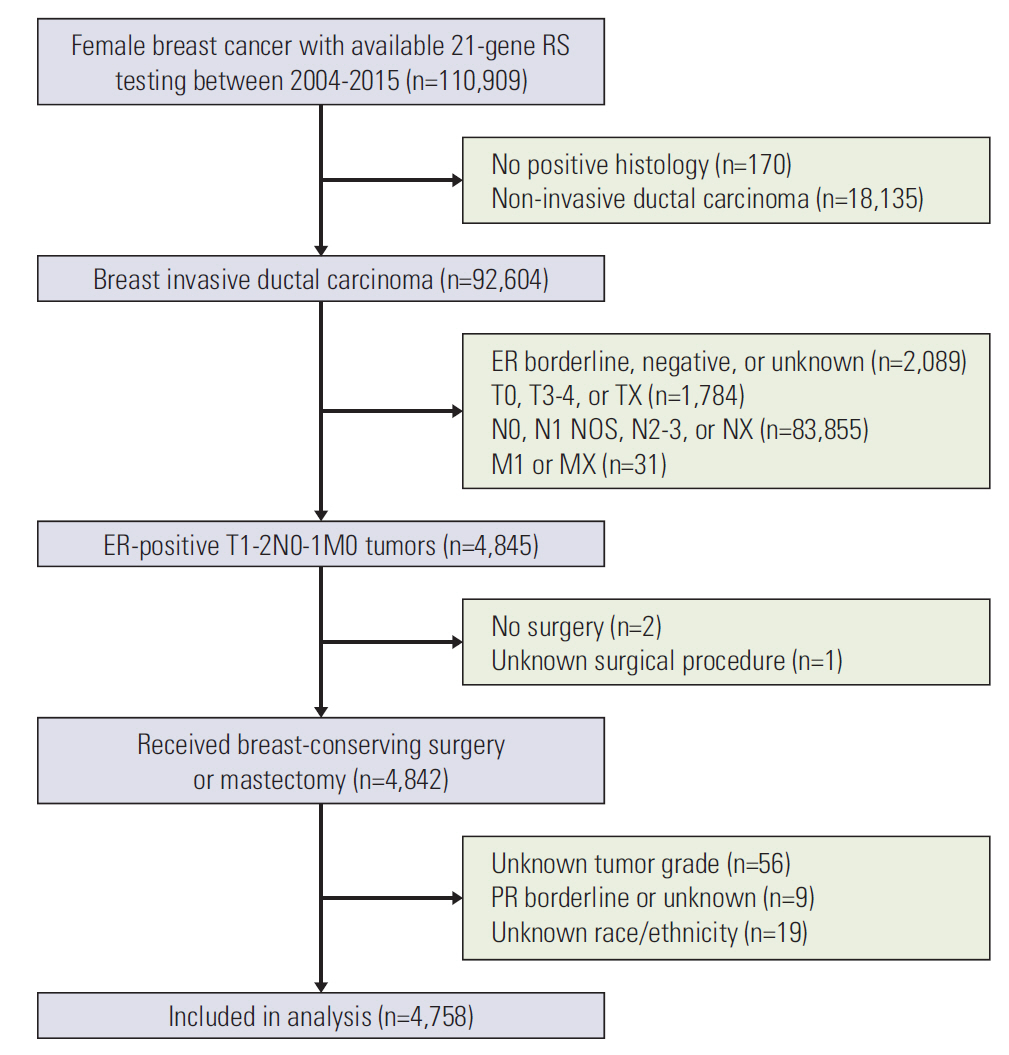
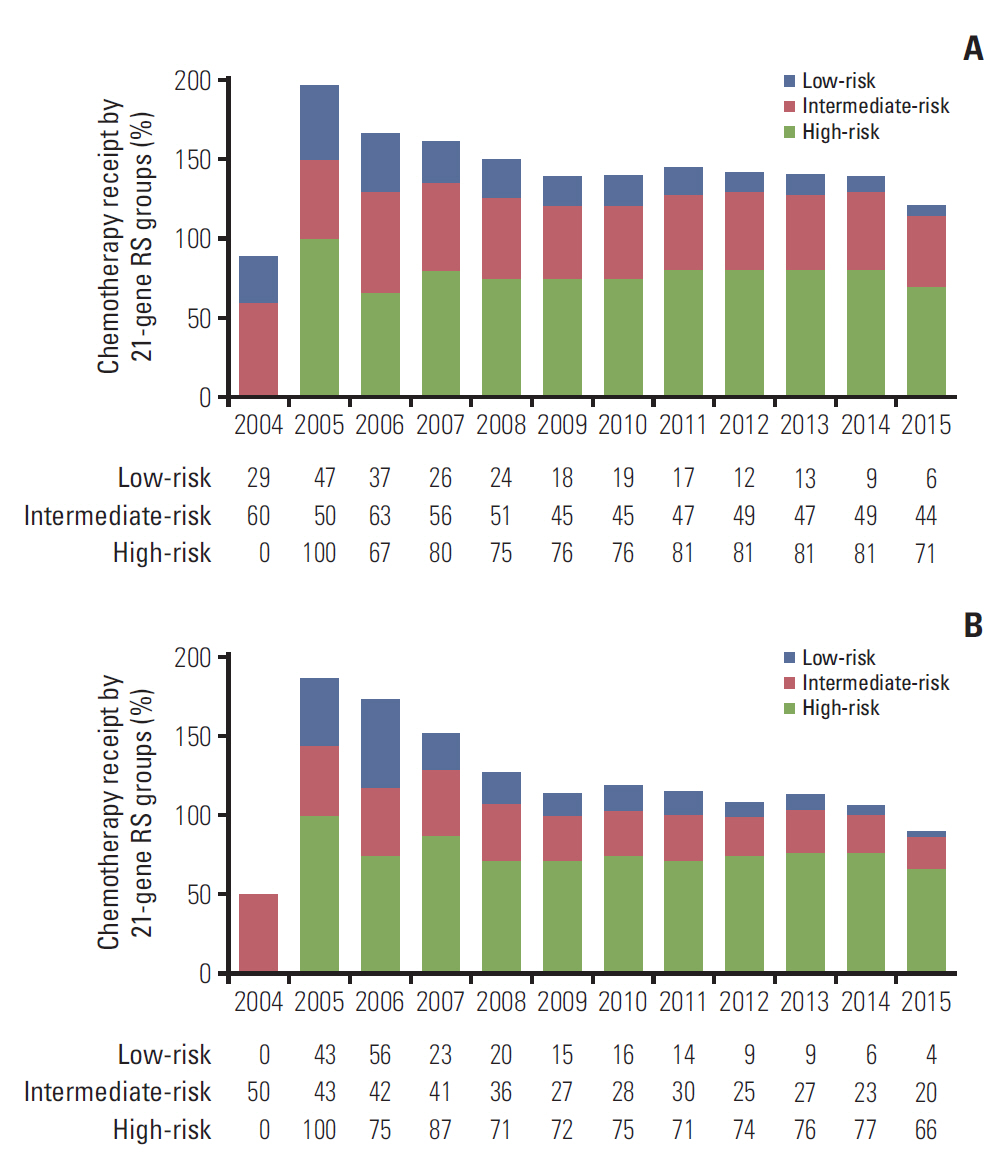
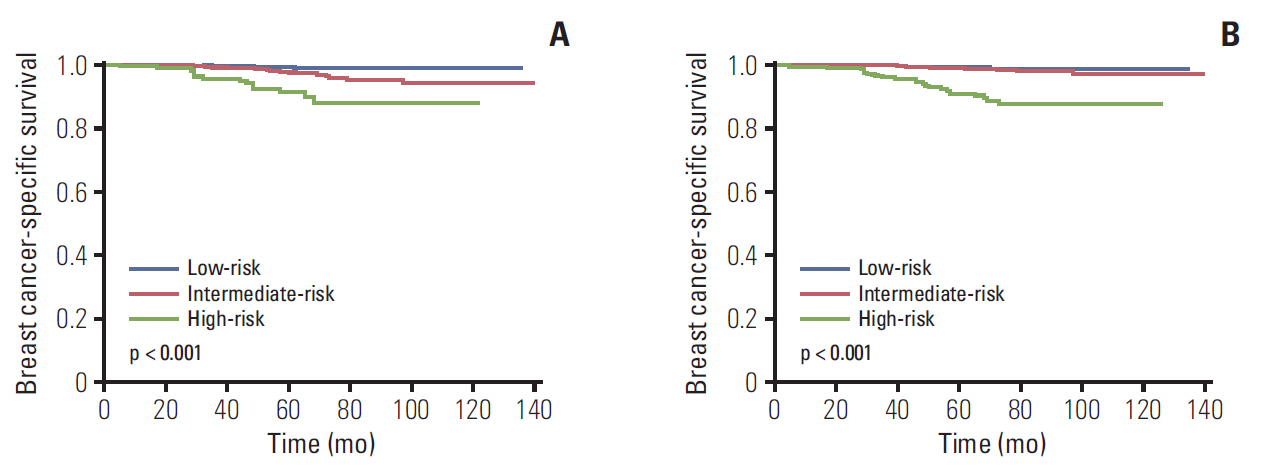
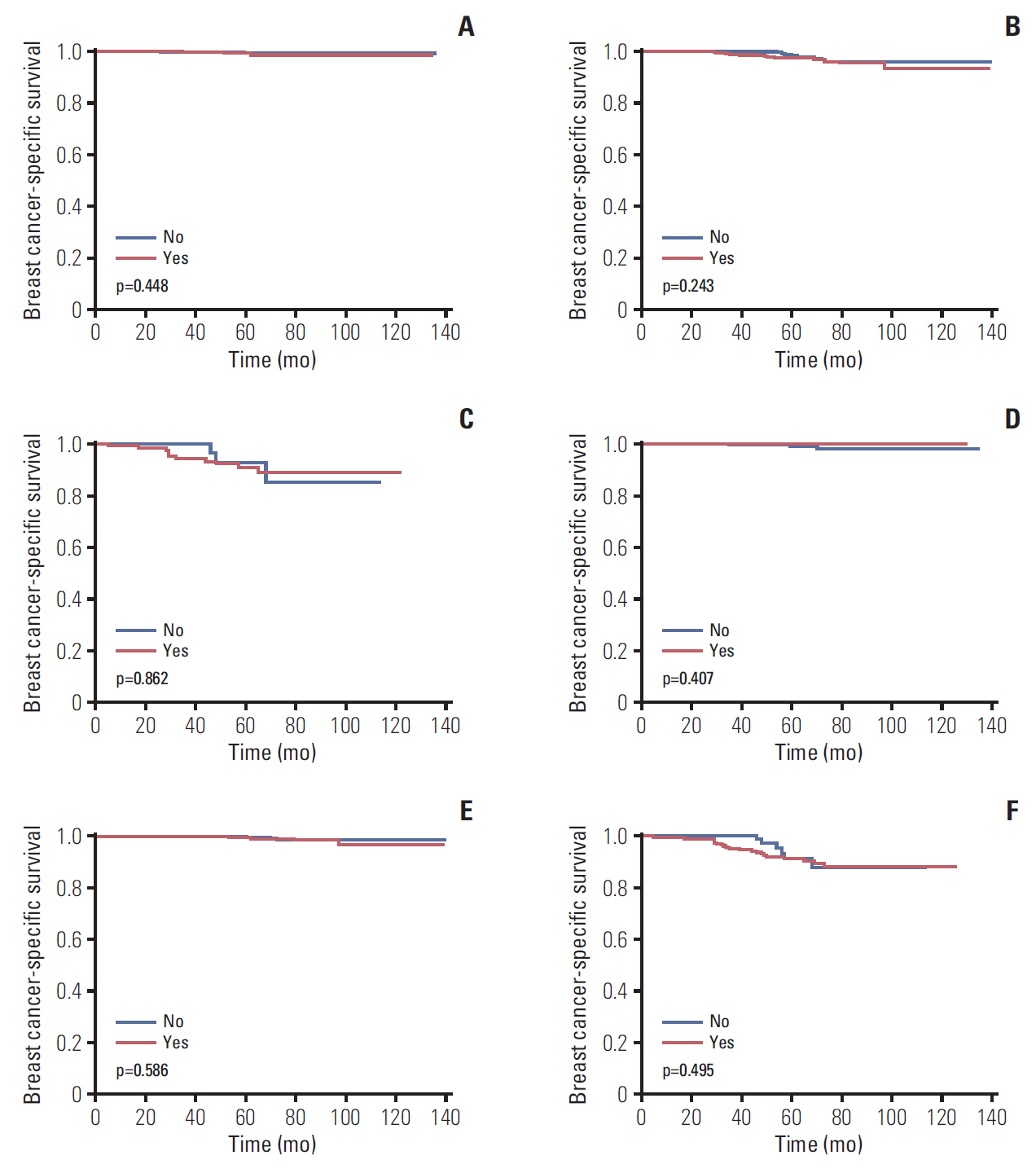
We identified 4,758 patients including 1,403 patients (29.5%) treated with adjuvant chemotherapy. In the traditional RS cutoffs, 2,831 (59.5%), 1,634 (34.3%), and 293 (6.2%) patients were in the low-, intermediate-, and high-risk RS groups, respectively. In 3,853 patients with human epidermal growth factor receptor-2 (HER2) status available, most patients were HER2-negative disease (98.3%). A higher RS was independently related to chemotherapy receipt, and 14.0%, 47.7%, and 77.8% of patients in the low-, intermediate-, and high-risk RS groups received chemotherapy, respectively. The multivariate analysis indicated that a higher RS was related to worse BCSS (p < 0.001). The 5-year BCSS rates were 99.3%, 97.4%, and 91.9% in patients with low-, intermediate-, and high-risk RS groups, respectively (p < 0.001). However, chemotherapy receipt did not correlate with better BCSS in low-, intermediate-, or high-risk RS groups. There were similar trends using Trial Assigning Individualized Options for Treatment RS cutoffs.
CONCLUSION
The 21-gene RS does predict outcome and impact on chemotherapy decision of N1mi breast IDC. Large cohort and long-term outcomes studies are needed to identify the effects of chemotherapy in N1mi patients by different 21-gene RS groups.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Maaskant AJ, van de Poll-Franse LV, Voogd AC, Coebergh JW, Tutein Nolthenius-Puylaert MC, Nieuwenhuijzen GA. Stage migration due to introduction of the sentinel node procedure: a population-based study. Breast Cancer Res Treat. 2009; 113:173–9.
Article2. van der Heiden-van der Loo M, Bezemer PD, Hennipman A, Siesling S, van Diest PJ, Bongers V, et al. Introduction of sentinel node biopsy and stage migration of breast cancer. Eur J Surg Oncol. 2006; 32:710–4.3. Salhab M, Patani N, Mokbel K. Sentinel lymph node micrometastasis in human breast cancer: an update. Surg Oncol. 2011; 20:e195–206.
Article4. Peethambaram PP, Hoskin TL, Day CN, Goetz MP, Habermann EB, Boughey JC. Use of 21-gene recurrence score assay to individualize adjuvant chemotherapy recommendations in ER+/HER2– node positive breast cancer-A National Cancer Database study. NPJ Breast Cancer. 2017; 3:41.
Article5. Montagna E, Viale G, Rotmensz N, Maisonneuve P, Galimberti V, Luini A, et al. Minimal axillary lymph node involvement in breast cancer has different prognostic implications according to the staging procedure. Breast Cancer Res Treat. 2009; 118:385–94.
Article6. Hansen NM, Grube B, Ye X, Turner RR, Brenner RJ, Sim MS, et al. Impact of micrometastases in the sentinel node of patients with invasive breast cancer. J Clin Oncol. 2009; 27:4679–84.
Article7. Langer I, Marti WR, Guller U, Moch H, Harder F, Oertli D, et al. Axillary recurrence rate in breast cancer patients with negative sentinel lymph node (SLN) or SLN micrometastases: prospective analysis of 150 patients after SLN biopsy. Ann Surg. 2005; 241:152–8.8. NCCN clinical Practice guidelines in oncology V.2.2018. Breast Cancer [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network;2018. [cited 2018 Oct 21]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.9. Andersson Y, Bergkvist L, Frisell J, de Boniface J. Long-term breast cancer survival in relation to the metastatic tumor burden in axillary lymph nodes. Breast Cancer Res Treat. 2018; 171:359–69.
Article10. Iqbal J, Ginsburg O, Giannakeas V, Rochon PA, Semple JL, Narod SA. The impact of nodal micrometastasis on mortality among women with early-stage breast cancer. Breast Cancer Res Treat. 2017; 161:103–15.
Article11. De Abreu FB, Schwartz GN, Wells WA, Tsongalis GJ. Personalized therapy for breast cancer. Clin Genet. 2014; 86:62–7.
Article12. Sabatier R, Goncalves A, Bertucci F. Personalized medicine: present and future of breast cancer management. Crit Rev Oncol Hematol. 2014; 91:223–33.
Article13. Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006; 24:3726–34.
Article14. Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010; 11:55–65.
Article15. Frazier TG, Fox KR, Smith JS, Laronga C, McSwain A, Paul D, et al. A retrospective study of the impact of 21-gene recurrence score assay on treatment choice in node positive micrometastatic breast cancer. Pharmaceuticals (Basel). 2015; 8:107–22.
Article16. Surveillance, Epidemiology, and End Results (SEER) Program. Oncotype DX database (2004-2015) [Internet]. Bethesda, MD: National Cancer Institute;2018. [cited 2018 Oct 21]. Available from: https://seer.cancer.gov/seerstat/databases/oncotypedx/index.html.17. Wong WB, Ramsey SD, Barlow WE, Garrison LP Jr, Veenstra DL. The value of comparative effectiveness research: projected return on investment of the RxPONDER trial (SWOG S1007). Contemp Clin Trials. 2012; 33:1117–23.
Article18. Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018; 379:111–21.
Article19. Wu SP, Tam M, Shaikh F, Lee A, Chun J, Schnabel F, et al. Post-mastectomy radiation therapy in breast cancer patients with nodal micrometastases. Ann Surg Oncol. 2018; 25:2620–31.
Article20. Turashvili G, Chou JF, Brogi E, Morrow M, Dickler M, Norton L, et al. 21-Gene recurrence score and locoregional recurrence in lymph node-negative, estrogen receptor-positive breast cancer. Breast Cancer Res Treat. 2017; 166:69–76.
Article21. Goodman CR, Seagle BL, Kocherginsky M, Donnelly ED, Shahabi S, Strauss JB. 21-Gene recurrence score assay predicts benefit of post-mastectomy radiotherapy in T1-2 N1 breast cancer. Clin Cancer Res. 2018; 24:3878–87.
Article22. Forissier V, Tallet A, Cohen M, Classe JM, Reyal F, Chopin N, et al. Is post-mastectomy radiation therapy contributive in pN0-1mi breast cancer patients? Results of a French multi-centric cohort. Eur J Cancer. 2017; 87:47–57.23. de Boer M, van Deurzen CH, van Dijck JA, Borm GF, van Diest PJ, Adang EM, et al. Micrometastases or isolated tumor cells and the outcome of breast cancer. N Engl J Med. 2009; 361:653–63.
Article24. Stemmer SM, Steiner M, Rizel S, Geffen DB, Nisenbaum B, Peretz T, et al. Clinical outcomes in ER+ HER2 -node-positive breast cancer patients who were treated according to the Recurrence Score results: evidence from a large prospectively designed registry. NPJ Breast Cancer. 2017; 3:32.
Article25. Henry NL, Somerfield MR, Abramson VG, Allison KH, Anders CK, Chingos DT, et al. Role of patient and disease factors in adjuvant systemic therapy decision making for earlystage, operable breast cancer: American Society of Clinical Oncology Endorsement of Cancer Care Ontario Guideline Recommendations. J Clin Oncol. 2016; 34:2303–11.
Article26. D'Arcy M, Fleming J, Robinson WR, Kirk EL, Perou CM, Troester MA. Race-associated biological differences among Luminal A breast tumors. Breast Cancer Res Treat. 2015; 152:437–48.27. Giordano SH, Lin YL, Kuo YF, Hortobagyi GN, Goodwin JS. Decline in the use of anthracyclines for breast cancer. J Clin Oncol. 2012; 30:2232–9.
Article28. Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med. 2015; 373:2005–14.
Article29. Krop I, Ismaila N, Andre F, Bast RC, Barlow W, Collyar DE, et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline Focused Update. J Clin Oncol. 2017; 35:2838–47.
Article30. Noone AM, Lund JL, Mariotto A, Cronin K, McNeel T, Deapen D, et al. Comparison of SEER treatment data with medicare claims. Med Care. 2016; 54:e55–64.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Clinical Impact of 21-Gene Recurrence Score on Treatment Decisions for Patients with Hormone Receptor-Positive Early Breast Cancer in Korea
- Detection of Micrometastases of Breast Cancer by Immunohistochemical Analysis of Cytokeratin
- Expression of MT-1 MMP, MMP2, MMP9 and TIMP2 mRNAs in Ductal Carcinoma in Situ and Invasive Ductal Carcinoma of the Breast
- Human Epidermal Growth Factor Receptor 2-Subtype Invasive Ductal Carcinoma Recurring as Basal-Human Epidermal Growth Factor Receptor 2-Subtype Squamous Cell Carcinoma
- Invasive Ductal Carcinoma of the Male Breast: A Case Report and Review of the Literature