Nat Prod Sci.
2019 Sep;25(3):233-237. 10.20307/nps.2019.25.3.233.
Diels-Alder Type Adducts from Hairy Root Cultures of Morus macroura
- Affiliations
-
- 1Chemistry Study Program, Faculty of Mathematics and Natural Sciences, Bandung Institute of Technology, Jl. Ganesha 10, 40132 Bandung, Indonesia. nizar@chem.itb.ac.id
- 2Department of Technical Biochemistry, Technical University of Dortmund, Emil-Figge-Str. 66, 44227 Dortmund, Germany.
- 3Research Center of Biotechnology, Indonesian Institute of Sciences (LIPI), Jl. Raya Bogor KM. 46 Cibinong 16911, Indonesia.
- KMID: 2459965
- DOI: http://doi.org/10.20307/nps.2019.25.3.233
Abstract
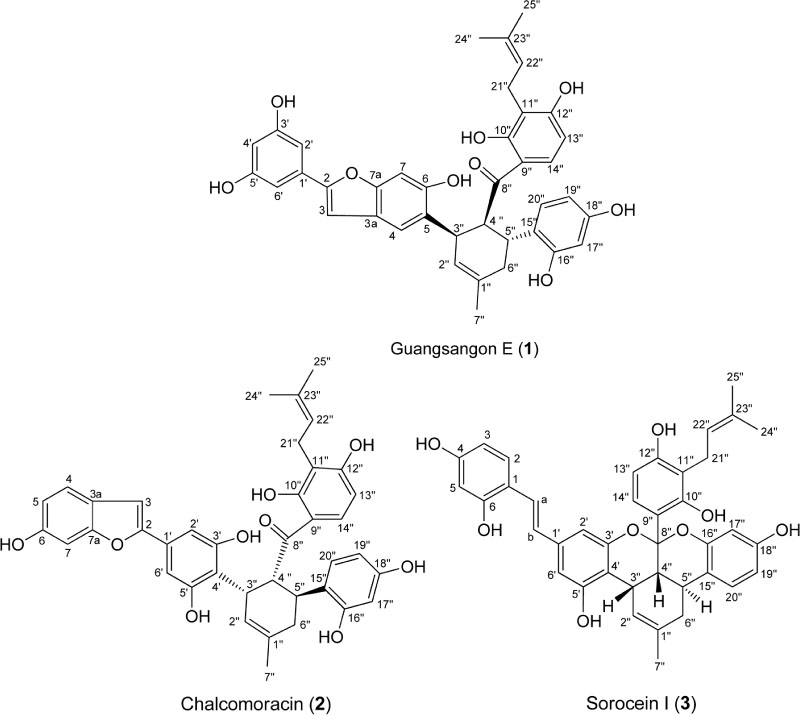
- Three Diels-Alder type adducts, guangsangon E (1), chalcomoracin (2) and sorocein I (3) were isolated from hairy root cultures of Morus macroura. The structures of the isolated compounds (1 - 3) were determined by spectroscopic method (NMR and MS), and spectral comparison to literature. Cytotoxic activities of the isolated compounds (1 - 3) were investigated against P-388 murine leukemia cell line. Guangsangon E (1) showed the most potent cytotoxicity against P-388 murine leukemia cell line with ICâ‚…â‚€ value of 2.75 ± 0.32 µg/mL. To the best of our knowledge, guangsangon E (1) and sorocein I (3) were reported for the first time from the tissue cultures of M. macroura.
Figure
Reference
-
1. Heyne K. Research and Development Agency. Ministry of Forestry. Indonesia: 1987. p. 659.2. Dai SJ, Ma ZB, Wu Y, Chen RY, Yu DQ. Phytochemistry. 2004; 65:3135–3141.3. Dai SJ, Mi ZM, Ma ZB, Li S, Chen RY, Yu DQ. Planta Med. 2004; 70:758–763.4. Dai SJ, Wu Y, Wang YH, He WY, Chen RY, Yu DQ. Chem Pharm Bull. 2004; 52:1190–1193.5. Dai SJ, Yu DQ. Nat Prod Res. 2006; 20:676–679.6. Wang YF, Xu L, Gao W, Niu L, Huang C, Yang P, Hu X. Planta Med. 2018; 84:336–343.7. Wang YF, Yu MH, Xu L, Niu L, Huang C, Xu H, Yang P, Hu X. Phytochem Lett. 2018; 26:149–153.8. Sun SG, Chen RY, Yu DQ. J Asian Nat Prod Res. 2001; 3:253–259.9. Syah YM, Achmad SA, Ghisalberti EL, Hakim EH, Iman MZ, Makmur L, Mujahiddin D. Fitoterapia. 2000; 71:630–635.10. Syah YM, Achmad SA, Ghisalberti EL, Hakim EH, Makmur L, Soekamto NH. J Chem Res. 2004; 2004:339–340.11. Happyana N, Mujahiddin D, Juliawaty LD, Makmur L, Achmad SA, Syah YM, Hakim EH. Proceeding of The International Seminar on Chemistry. Indonesia: Universitas Padjadjaran;2008. p. 257–261.12. Kang J, Chen RY, Yu DQ. Planta Med. 2006; 72:52–59.13. Takasugi M, Nagao S, Masamune T, Shirata A, Takahashi K. Chem Lett. 1980; 9:1573–1576.14. Ferrari F, Delle Monache F. Fitoterapia. 2001; 72:301–303.15. Sahidin Hakim EH, Juliawaty LD, Syah YM, bin Din L, Ghisalberti EL, Latip J, Said IM, Achmad SA. Z Naturforsch C. 2005; 60:723–727.16. Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, Abbott BJ, Mayo JG, Shoemaker RH, Boyd MR. Cancer Res. 1988; 48:589–601.17. Kim YJ, Sohn MJ, Kim WG. Biol Pharm Bull. 2012; 35:791–795.18. Zhang QJ, Tang YB, Chen RY, Yu DQ. Chem Biodivers. 2007; 4:1533–1540.19. Han H, Chou CC, Li R, Liu J, Zhang L, Zhu W, Hu J, Yang B. Tian J Sci Rep. 2018; 8:9566.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hairy cell transformation of human peripheral blood lymphocytes by Coxiella burnetii
- A Case of Black Hairy Tongue
- Measurement of Serum Levels of 25-Hydroxyvitamin D3 and 25-Hydroxyvitamin D2 Using Diels-Alder Derivatization and Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry
- A Case of Black Hairy Tongue
- A Case of Black Hairy Tongue following the Use of Psychotropic Agents