J Pathol Transl Med.
2019 Sep;53(5):280-288. 10.4132/jptm.2019.05.13.
High Expression of Galectin-1, VEGF and Increased Microvessel Density Are Associated with MELF Pattern in Stage I-III Endometrioid Endometrial Adenocarcinoma
- Affiliations
-
- 1Department of Pathology, Gomel State Medical University, Gomel, Belarus.
- 2Department of Pathology, Gomel State Clinical Oncological Dispensary, Gomel, Belarus.
- 3Department of Pathology, Grodno Regional Clinical Bureau of Pathology, Grodno, Belarus.
- 4Institute of Biomedical and Clinical Science, University of Exeter Medical School, Exeter, Devon, UK. J.L.Whatmore@exeter.ac.uk
- 5William Harvey Research Institute, Barts & The London School of Medicine & Dentistry Queen Mary University of London, London, UK. z.pranjol@qmul.ac.uk
- KMID: 2459576
- DOI: http://doi.org/10.4132/jptm.2019.05.13
Abstract
- BACKGROUND
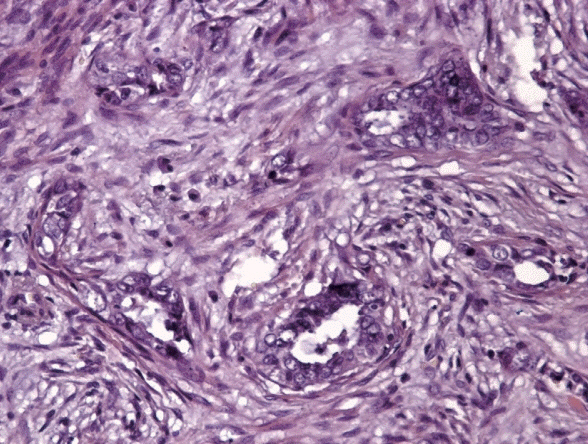
In this study, we investigate the expression of markers of angiogenesis and microvessel density (MVD) in cases of microcystic, elongated and fragmented (MELF) pattern, with its prognostic role in the survival of endometrioid endometrial adenocarcinomas (EA) patients.
METHODS
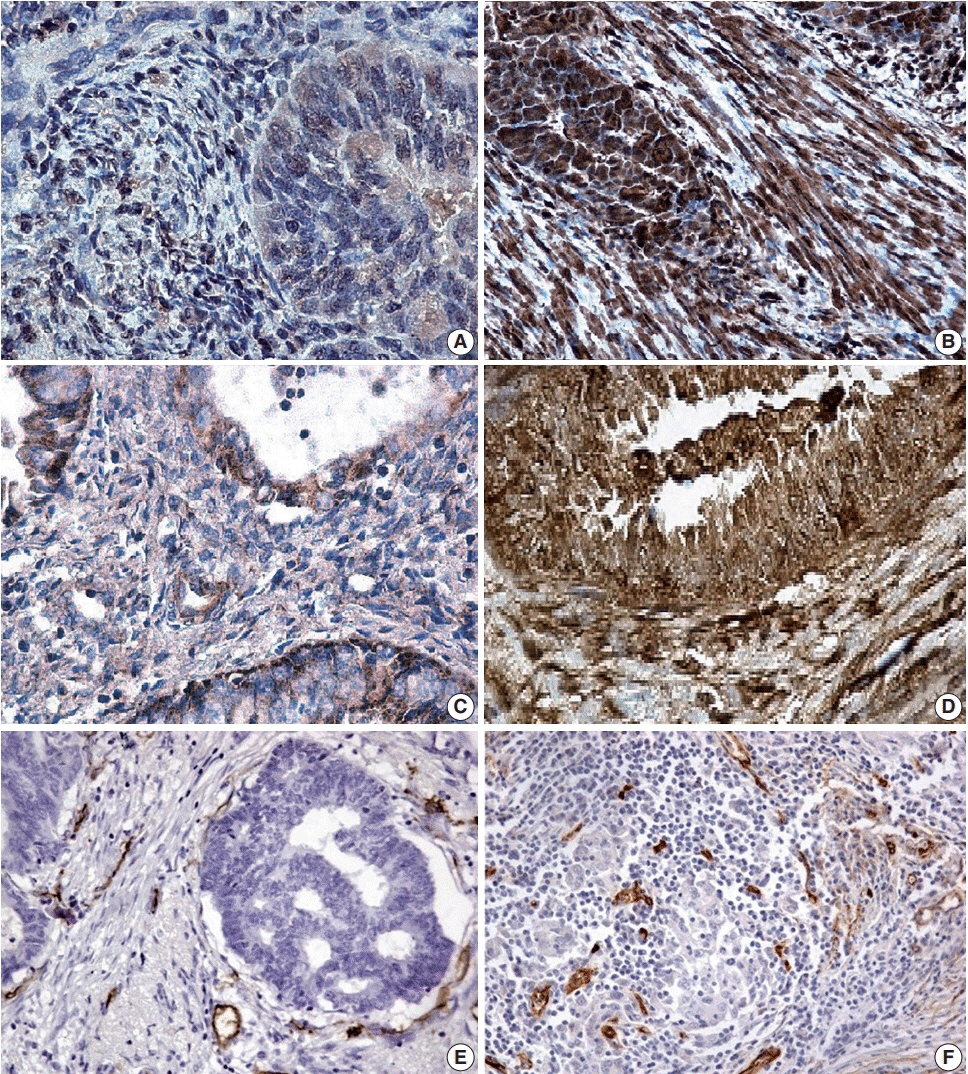
In this study, 100 cases of EA, 49 cases with MELF pattern and 51 without, were immunohistochemically stained for galectin-1, vascular endothelial growth factor (VEGF), and MVD. Morphometry and statistical (univariate and multivariate) analyses were performed to assess overall survival (OS) and disease-free survival.
RESULTS
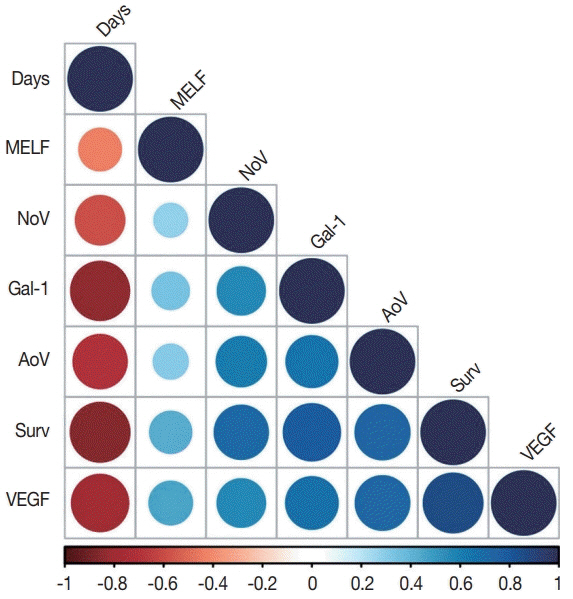
The expression of VEGF (p<.001) and galectin-1 (p<.001), as well as MVD area (p<.001) and number of vessels/mm² (p<.050), were significantly higher in the +MELF pattern group compared to the -MELF group. A low negative correlation between MELF-pattern and the number of days of survival (p<.001, r=-0.47) was also found. A low positive correlation of MELF-pattern with galectin-1 expression (p<.001, r=0.39), area of vessels/mm² (p<.001, r=0.36), outcome of EA (p<.001, r=0.42) and VEGF expression (p<.001, r=0.39) suggests potential pathological relevance of these factors in the prognosis of EA. A univariate survival analysis indicated a role for all parameters of survival. Multivariate Cox proportional hazard regression analysis revealed that only area of vessels/mm² (hazard ratio [HR], 1.018; 95% confidence interval [CI], 1.002 to 1.033), galectin-1 (HR, 1.049; 95% CI, 1.025 to 1.074) and VEGF (HR, 1.049; 95% CI, 1.022 to 1.077) play key roles in OS.
CONCLUSIONS
This study reports an increase in MVD, VEGF and galectin-1 expression in EA with MELF pattern and suggests that MELF pattern, along with the angiogenic profile, may be a prognostic factor in EA.
MeSH Terms
Figure
Reference
-
1. Horn LC, Meinel A, Handzel R, Einenkel J. Histopathology of endometrial hyperplasia and endometrial carcinoma: an update. Ann Diagn Pathol. 2007; 11:297–311.2. Banas T, Pitynski K, Okon K, Winiarska A. Non-endometrioid and high-grade endometrioid endometrial cancers show DNA fragmentation factor 40 (DFF40) and B-cell lymphoma 2 protein (BCL2) underexpression, which predicts disease-free and overall survival, but not DNA fragmentation factor 45 (DFF45) underexpression. BMC Cancer. 2018; 18:418.
Article3. Reeves KW, Carter GC, Rodabough RJ, et al. Obesity in relation to endometrial cancer risk and disease characteristics in the Women’s Health Initiative. Gynecol Oncol. 2011; 121:376–82.
Article4. SGO Clinical Practice Endometrial Cancer Working Group, Burke WM, Orr J, et al. Endometrial cancer: a review and current management strategies: part II. Gynecol Oncol. 2014; 134:393–402.
Article5. Murray SK, Young RH, Scully RE. Unusual epithelial and stromal changes in myoinvasive endometrioid adenocarcinoma: a study of their frequency, associated diagnostic problems, and prognostic significance. Int J Gynecol Pathol. 2003; 22:324–33.
Article6. Sanci M, Güngördük K, Gülseren V, et al. MELF pattern for predicting lymph node involvement and survival in grade I-II endometrioid-type endometrial cancer. Int J Gynecol Pathol. 2018; 37:17–21.
Article7. Kihara A, Yoshida H, Watanabe R, et al. Clinicopathologic association and prognostic value of microcystic, elongated, and fragmented (MELF) pattern in endometrial endometrioid carcinoma. Am J Surg Pathol. 2017; 41:896–905.
Article8. Pelletier MP, Trinh VQ, Stephenson P, et al. Microcystic, elongated, and fragmented pattern invasion is mainly associated with isolated tumor cell pattern metastases in International Federation of Gynecology and Obstetrics grade I endometrioid endometrial cancer. Hum Pathol. 2017; 62:33–9.
Article9. Stewart CJ, Brennan BA, Leung YC, Little L. MELF pattern invasion in endometrial carcinoma: association with low grade, myoinvasive endometrioid tumours, focal mucinous differentiation and vascular invasion. Pathology. 2009; 41:454–9.
Article10. Zinovkin DA, Pranjol MZI, Petrenyov DR, Nadyrov EA, Savchenko OG. The potential roles of MELF-pattern, microvessel density, and VEGF expression in survival of patients with endometrioid endometrial carcinoma: a morphometrical and immunohistochemical analysis of 100 cases. J Pathol Transl Med. 2017; 51:456–62.
Article11. Zinovkin D, Pranjol MZ. Tumor-infiltrated lymphocytes, macrophages, and dendritic cells in endometrioid adenocarcinoma of corpus uteri as potential prognostic factors: an immunohistochemical study. Int J Gynecol Cancer. 2016; 26:1207–12.
Article12. Zhu X, Wang K, Zhang K, et al. Galectin-1 knockdown in carcinomaassociated fibroblasts inhibits migration and invasion of human MDA-MB-231 breast cancer cells by modulating MMP-9 expression. Acta Biochim Biophys Sin (Shanghai). 2016; 48:462–7.
Article13. Jeschke U, Walzel H, Mylonas I, et al. The human endometrium expresses the glycoprotein mucin-1 and shows positive correlation for Thomsen-Friedenreich epitope expression and galectin-1 binding. J Histochem Cytochem. 2009; 57:871–81.
Article14. Sandberg TP, Oosting J, van Pelt GW, Mesker WE, Tollenaar R, Morreau H. Molecular profiling of colorectal tumors stratified by the histological tumor-stroma ratio: increased expression of galectin-1 in tumors with high stromal content. Oncotarget. 2018; 9:31502–15.15. Zinovkin DA, Pranjol MZ, Bilsky IA, Zmushko VA. Tumor-associated T-lymphocytes and macrophages are decreased in endometrioid endometrial carcinoma with MELF-pattern stromal changes. Cancer Microenviron. 2018; 11:107–14.
Article16. Albini A, Bruno A, Noonan DM, Mortara L. Contribution to tumor angiogenesis from innate immune cells within the tumor microenvironment: implications for immunotherapy. Front Immunol. 2018; 9:527.
Article17. Melincovici CS, Bos¸ca AB, S¸us¸man S, et al. Vascular endothelial growth factor (VEGF): key factor in normal and pathological angiogenesis. Rom J Morphol Embryol. 2018; 59:455–67.18. Tao L, Huang G, Song H, Chen Y, Chen L. Cancer associated fibroblasts: an essential role in the tumor microenvironment. Oncol Lett. 2017; 14:2611–20.
Article19. Li YL, Zhao H, Ren XB. Relationship of VEGF/VEGFR with immune and cancer cells: staggering or forward? Cancer Biol Med. 2016; 13:206–14.
Article20. Miyata Y, Sakai H. Reconsideration of the clinical and histopathological significance of angiogenesis in prostate cancer: Usefulness and limitations of microvessel density measurement. Int J Urol. 2015; 22:806–15.
Article21. Jilaveanu LB, Puligandla M, Weiss SA, et al. Tumor microvessel density as a prognostic marker in high-risk renal cell carcinoma patients treated on ECOG-ACRIN E2805. Clin Cancer Res. 2018; 24:217–23.
Article22. Hu X, Liu H, Ye M, Zhu X. Prognostic value of microvessel density in cervical cancer. Cancer Cell Int. 2018; 18:152.
Article23. Mezheyeuski A, Nerovnya A, Bich T, Tur G, Ostman A, Portyanko A. Inter- and intra-tumoral relationships between vasculature characteristics, GLUT1 and budding in colorectal carcinoma. Histol Histopathol. 2015; 30:1203–11.24. Joehlin-Price AS, McHugh KE, Stephens JA, et al. The microcystic, elongated, and fragmented (MELF) pattern of invasion: a single institution report of 464 consecutive FIGO grade 1 endometrial endometrioid adenocarcinomas. Am J Surg Pathol. 2017; 41:49–55.25. Kamili NA, Arthur CM, Gerner-Smidt C, et al. Key regulators of galectin-glycan interactions. Proteomics. 2016; 16:3111–25.
Article26. Chen J, Tang D, Wang S, et al. High expressions of galectin-1 and VEGF are associated with poor prognosis in gastric cancer patients. Tumour Biol. 2014; 35:2513–9.
Article27. Wu R, Wu T, Wang K, et al. Prognostic significance of galectin-1 expression in patients with cancer: a meta-analysis. Cancer Cell Int. 2018; 18:108.
Article28. Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013; 13:871–82.
Article29. Wang JZ, Xiong YJ, Man GC, Chen XY, Kwong J, Wang CC. Clinicopathological and prognostic significance of blood microvessel density in endometrial cancer: a meta-analysis and subgroup analysis. Arch Gynecol Obstet. 2018; 297:731–40.
Article30. Prodromidou A, Vorgias G, Bakogiannis K, Kalinoglou N, Iavazzo C. MELF pattern of myometrial invasion and role in possible endometrial cancer diagnostic pathway: a systematic review of the literature. Eur J Obstet Gynecol Reprod Biol. 2018; 230:147–52.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Potential Roles of MELF-Pattern, Microvessel Density, and VEGF Expression in Survival of Patients with Endometrioid Endometrial Carcinoma: A Morphometrical and Immunohistochemical Analysis of 100 Cases
- Microvessel Density and Expressions of bcl-2, p53, and Vascular Endothelial Growth Factor in Endometrial Carcinoma
- Immunohistochemical Study of COX-2, VEGF, CD34 and MMP-9 Expression in Colonic Adenocarcinoma
- VEGF Expression and Microvessel Density in Oral Squamous Cell Carcinomas
- Expression of Vascular Endothelial Growth Factor (VEGF) and Microvessel Density in Hepatocellular Carcinoma