Korean J Ophthalmol.
2019 Oct;33(5):406-413. 10.3341/kjo.2019.0022.
Evaluation of Metabolite Changes in the Occipital Cortex of Patients with Idiopathic Infantile Nystagmus or Bilateral Ametropic Amblyopia by Magnetic Resonance Spectroscopy
- Affiliations
-
- 1Department of Ophthalmology, Kiziltepe Private Ipekyolu Hospital, Mardin, Turkey.
- 2Department of Ophthalmology, Haseki Training and Research Hospital, University of Health Sciences, Istanbul, Turkey. ercanozy@hotmail.com
- 3Department of Ophthalmology, Private Goznuru Eye Hospital, Gaziantep, Turkey.
- 4Department of Ophthalmology, Baskent University, Izmir, Turkey.
- 5Department of Ophthalmology, Ataturk State Hospital, Antalya, Turkey.
- KMID: 2459521
- DOI: http://doi.org/10.3341/kjo.2019.0022
Abstract
- PURPOSE
To evaluate the effects of idiopathic infantile nystagmus (IN) and bilateral ametropic amblyopia on metabolites in the occipital cortex by magnetic resonance spectroscopy.
METHODS
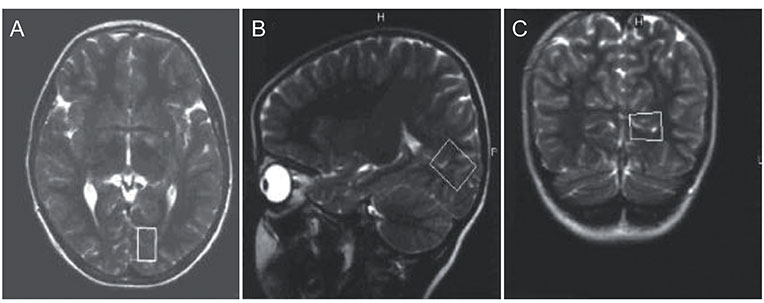
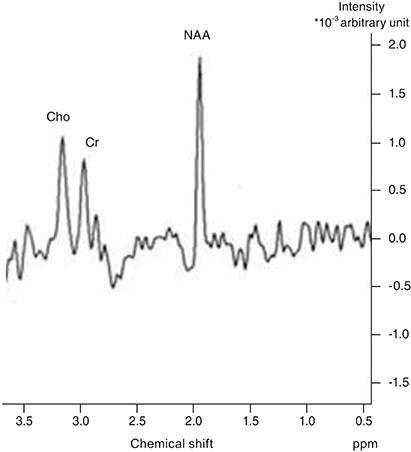
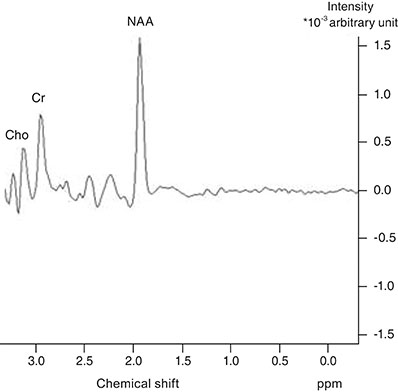
The children included in this prospective study were divided into three groups. Group 1 consisted of 11 patients with idiopathic IN, group 2 consisted of 10 patients with bilateral ametropic amblyopia and group 3 consisted of nine normal children. A single-voxel magnetic resonance spectroscopy examination was performed by placing a region of interest on the occipital cortex of each participant. N-acetyl aspartate (NAA), creatine (Cr) and choline (Cho) concentrations were measured in the occipital cortex. This was followed by calculating and comparing the NAA/Cr and Cho/Cr ratios between the three groups. The Kruskal-Wallis test, Mann-Whitney U-test, and chi-square test were used for statistical analysis.
RESULTS
There was no statistically significant difference in NAA/Cr ratios between patients with idiopathic IN and normal children, but there was a statistically significant difference between these groups when Cho/Cr ratios were compared; the ratio was higher in the idiopathic IN group. There were no statistically significant differences in NAA/Cr or Cho/Cr ratios between patients with bilateral ametropic amblyopia and normal children.
CONCLUSIONS
Our findings suggest that the neurochemical profile of the occipital cortex is partially affected by idiopathic IN, but not by bilateral ametropic amblyopia.
MeSH Terms
Figure
Reference
-
1. Papageorgiou E, McLean RJ, Gottlob I. Nystagmus in childhood. Pediatr Neonatol. 2014; 55:341–351.
Article2. Penix K, Swanson MW, DeCarlo DK. Nystagmus in pediatric patients: interventions and patient-focused perspectives. Clin Ophthalmol. 2015; 9:1527–1536.3. Khanna S, Dell’Osso LF. The diagnosis and treatment of infantile nystagmus syndrome (INS). ScientificWorldJournal. 2006; 6:1385–1397.
Article4. Sarvananthan N, Surendran M, Roberts EO, et al. The prevalence of nystagmus: the Leicestershire nystagmus survey. Invest Ophthalmol Vis Sci. 2009; 50:5201–5206.
Article5. Abadi RV. Mechanisms underlying nystagmus. J R Soc Med. 2002; 95:231–234.
Article6. Magdalene D, Bhattacharjee H, Choudhury M, et al. Community outreach: an indicator for assessment of prevalence of amblyopia. Indian J Ophthalmol. 2018; 66:940–944.
Article7. Joly O, Franko E. Neuroimaging of amblyopia and binocular vision: a review. Front Integr Neurosci. 2014; 8:62.
Article8. Firat PG, Ozsoy E, Demirel S, et al. Evaluation of peripapillary retinal nerve fiber layer, macula and ganglion cell thickness in amblyopia using spectral optical coherence tomography. Int J Ophthalmol. 2013; 6:90–94.9. Gujar SK, Maheshwari S, Bjorkman-Burtscher I, Sundgren PC. Magnetic resonance spectroscopy. J Neuroophthalmol. 2005; 25:217–226.
Article10. Simmons ML, Frondoza CG, Coyle JT. Immunocytochemical localization of N-acetyl-aspartate with monoclonal antibodies. Neuroscience. 1991; 45:37–45.
Article11. Block W, Traber F, Flacke S, et al. In-vivo proton MR-spectroscopy of the human brain: assessment of N-acetylaspartate (NAA) reduction as a marker for neurodegeneration. Amino Acids. 2002; 23:317–323.
Article12. Demougeot C, Garnier P, Mossiat C, et al. N-Acetylaspartate, a marker of both cellular dysfunction and neuronal loss: its relevance to studies of acute brain injury. J Neurochem. 2001; 77:408–415.
Article13. Boucard CC, Hoogduin JM, van der Grond J, Cornelissen FW. Occipital proton magnetic resonance spectroscopy (1H-MRS) reveals normal metabolite concentrations in retinal visual field defects. PLoS One. 2007; 2:e222.
Article14. Chan YL, Yeung DK, Leung SF, Cao G. Proton magnetic resonance spectroscopy of late delayed radiation-induced injury of the brain. J Magn Reson Imaging. 1999; 10:130–137.
Article15. Schuff N, Amend D, Ezekiel F, et al. Changes of hippocampal N-acetyl aspartate and volume in Alzheimer’s disease. A proton MR spectroscopic imaging and MRI study. Neurology. 1997; 49:1513–1521.
Article16. Zhang Y, Chen X, Wen G, et al. Proton magnetic resonance spectroscopy ((1)H-MRS) reveals geniculocalcarine and striate area degeneration in primary glaucoma. PLoS One. 2013; 8:e73197.
Article17. Weaver KE, Richards TL, Saenz M, et al. Neurochemical changes within human early blind occipital cortex. Neuroscience. 2013; 252:222–233.
Article18. Ozsoy E, Doganay S, Dogan M, et al. Evaluation of metabolite changes in visual cortex in diabetic retinopathy by MR-spectroscopy. J Diabetes Complications. 2012; 26:241–245.
Article19. Sahin I, Alkan A, Keskin L, et al. Evaluation of in vivo cerebral metabolism on proton magnetic resonance spectroscopy in patients with impaired glucose tolerance and type 2 diabetes mellitus. J Diabetes Complications. 2008; 22:254–260.
Article20. Behar KL, den Hollander JA, Stromski ME, et al. High-resolution 1H nuclear magnetic resonance study of cerebral hypoxia in vivo. Proc Natl Acad Sci U S A. 1983; 80:4945–4948.
Article21. Berkowitz BA, Bansal N, Wilson CA. Non-invasive measurement of steady-state vitreous lactate concentration. NMR Biomed. 1994; 7:263–268.
Article22. Rucker JC, Biousse V, Mao H, et al. Detection of lactate in the human vitreous body using proton magnetic resonance spectroscopy. Arch Ophthalmol. 2003; 121:909–911.
Article23. Zhao H, Huang XF, Zheng ZL, et al. Molecular genetic analysis of patients with sporadic and X-linked infantile nystagmus. BMJ Open. 2016; 6:e010649.
Article24. Watkins RJ, Patil R, Goult BT, et al. A novel interaction between FRMD7 and CASK: evidence for a causal role in idiopathic infantile nystagmus. Hum Mol Genet. 2013; 22:2105–2118.
Article25. Barrett BT, Bradley A, McGraw PV. Understanding the neural basis of amblyopia. Neuroscientist. 2004; 10:106–117.
Article26. Barrett BT, Bradley A, Candy TR. The relationship between anisometropia and amblyopia. Prog Retin Eye Res. 2013; 36:120–158.
Article27. Mangia S, Kumar AF, Moheet AA, et al. Neurochemical profile of patients with type 1 diabetes measured by 1H-MRS at 4 T. J Cereb Blood Flow Metab. 2013; 33:754–759.
Article28. Coullon GS, Emir UE, Fine I, et al. Neurochemical changes in the pericalcarine cortex in congenital blindness attributable to bilateral anophthalmia. J Neurophysiol. 2015; 114:1725–1733.
Article29. Castillo M, Kwock L, Mukherji SK. Clinical applications of proton MR spectroscopy. AJNR Am J Neuroradiol. 1996; 17:1–15.30. Maheshwari SR, Fatterpekar GM, Castillo M, Mukherji SK. Proton MR spectroscopy of the brain. Semin Ultrasound CT MR. 2000; 21:434–451.
Article31. Hufner K, Stephan T, Flanagin VL, et al. Cerebellar and visual gray matter brain volume increases in congenital nystagmus. Front Neurol. 2011; 2:60.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Electrooculographic Study of Latent Nystagmus
- Analysis and Treatment of Axial Ametropic AnisPmetropia
- A Case of Atypical Latent Nystagmus Associated with Infantile Nystagmus
- Magnetic Resonance Imaging of Idiopathic Herniation of the Lingual Gyrus: a Case Report
- The Role of Insular Cortex in Response to Group Therapy in Vaginismus Patients: Magnetic Resonance Spectroscopy Study