Allergy Asthma Immunol Res.
2019 Nov;11(6):763-778. 10.4168/aair.2019.11.6.763.
Future Risks in Patients With Severe Asthma
- Affiliations
-
- 1Department of Allergy and Clinical Immunology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. swj0126@amc.seoul.kr
- 2Department of Internal Medicine, Pusan National University School of Medicine, Busan, Korea.
- 3College of Nursing, Research Institute of Nursing Science, Kyungpook National University, Daegu, Korea.
- 4National Heart & Lung Institute, Imperial College London & Royal Brompton and Harefield NHS Trust, London, United Kingdom.
- KMID: 2459182
- DOI: http://doi.org/10.4168/aair.2019.11.6.763
Abstract
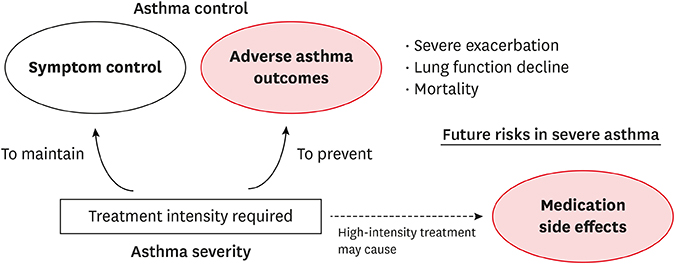
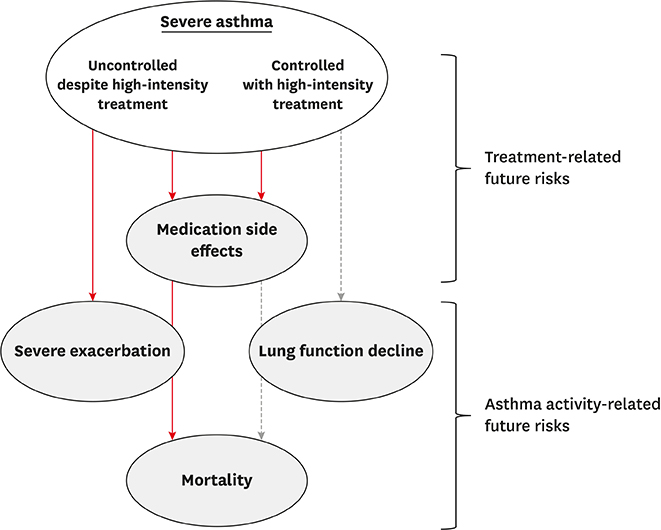
- A major burden of severe asthma is the future risk of adverse health outcomes. Patients with severe asthma are prone to serious exacerbation and deterioration of lung function and may experience side effects of medications such as oral corticosteroids (OCSs). However, such future risk is not easily measurable in daily clinical practice. In particular, currently available tools to measure asthma control and asthma-related quality of life incompletely predict the future risk of medication-related morbidity. This is a significant issue in asthma management. This review summarizes the current evidence of future risk in patients with severe asthma. As future risk is poorly perceived by controlled asthmatics, our review focuses on the risk in patients with "˜controlled' severe asthma. Of note, it is likely that long-term OCS therapy may not prevent future asthma progression, including lung function decline. In addition, the risk of drug side effects increases even during low-dose OCS therapy. Thus, novel treatments are highly desirable for reducing future risks without any loss of asthma control.
MeSH Terms
Figure
Cited by 1 articles
-
Increasing Prevalence and Mortality of Asthma With Age in Korea, 2002–2015: A Nationwide, Population-Based Study
Eunyoung Lee, Anhye Kim, Young-Min Ye, Sang-Eun Choi, Hae-Sim Park
Allergy Asthma Immunol Res. 2020;12(3):467-484. doi: 10.4168/aair.2020.12.3.467.
Reference
-
1. Wenzel SE, Brillhart S, Nowack K. An invisible disease: severe asthma is more than just “bad asthma”. Eur Respir J. 2017; 50:1701109.
Article2. Foster JM, McDonald VM, Guo M, Reddel HK. “I have lost in every facet of my life”: the hidden burden of severe asthma. Eur Respir J. 2017; 50:1700765.
Article3. Eassey D, Reddel HK, Foster JM, Kirkpatrick S, Locock L, Ryan K, et al. “…I've said I wish I was dead, you'd be better off without me”: a systematic review of people's experiences of living with severe asthma. J Asthma. 2019; 56:311–322.
Article4. Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014; 43:343–373.5. Hyland ME, Whalley B, Jones RC, Masoli M. A qualitative study of the impact of severe asthma and its treatment showing that treatment burden is neglected in existing asthma assessment scales. Qual Life Res. 2015; 24:631–639.
Article6. Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, Casale TB, et al. A new perspective on concepts of asthma severity and control. Eur Respir J. 2008; 32:545–554.
Article7. Bateman ED, Bousquet J, Braunstein GL. Is overall asthma control being achieved? A hypothesis-generating study. Eur Respir J. 2001; 17:589–595.
Article8. Papaioannou AI, Kostikas K, Zervas E, Kolilekas L, Papiris S, Gaga M. Control of asthma in real life: still a valuable goal? Eur Respir Rev. 2015; 24:361–369.
Article9. Hekking PP, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015; 135:896–902.
Article10. Mincheva R, Ekerljung L, Bossios A, Lundbäck B, Lötvall J. High prevalence of severe asthma in a large random population study. J Allergy Clin Immunol. 2018; 141:2256–2264.e2.
Article11. Chen S, Golam S, Myers J, Bly C, Smolen H, Xu X. Systematic literature review of the clinical, humanistic, and economic burden associated with asthma uncontrolled by GINA Steps 4 or 5 treatment. Curr Med Res Opin. 2018; 34:2075–2088.
Article12. Silkoff PE, Laviolette M, Singh D, FitzGerald JM, Kelsen S, Backer V, et al. Longitudinal stability of asthma characteristics and biomarkers from the Airways Disease Endotyping for Personalized Therapeutics (ADEPT) study. Respir Res. 2016; 17:43.
Article13. Kim MH, Kim SH, Park SY, Ban GY, Kim JH, Jung JW, et al. Characteristics of adult severe refractory asthma in Korea analyzed from the severe asthma registry. Allergy Asthma Immunol Res. 2019; 11:43–54.
Article14. Hermosa JL, Sánchez CB, Rubio MC, Mínguez MM, Walther JL. Factors associated with the control of severe asthma. J Asthma. 2010; 47:124–130.
Article15. Díez JM, Barcina C, Muñoz M, Leal M. Control of persistent asthma in Spain: associated factors. J Asthma. 2008; 45:740–746.
Article16. Turktas H, Mungan D, Uysal MA, Oguzulgen K. Turkish Asthma Control Survey Study Group. Determinants of asthma control in tertiary level in Turkey: a cross-sectional multicenter survey. J Asthma. 2010; 47:557–562.
Article17. Yii AC, Tan JH, Lapperre TS, Chan AK, Low SY, Ong TH, et al. Long-term future risk of severe exacerbations: distinct 5-year trajectories of problematic asthma. Allergy. 2017; 72:1398–1405.
Article18. Magnoni MS, Latorre M, Bettoncelli G, Sanchez-Herrero MG, Lopez A, Calvo E, et al. Asthma control in primary care: the results of an observational cross-sectional study in Italy and Spain. World Allergy Organ J. 2017; 10:13.
Article19. Chipps BE, Haselkorn T, Paknis B, Ortiz B, Bleecker ER, Kianifard F, et al. More than a decade follow-up in patients with severe or difficult-to-treat asthma: The Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens (TENOR) II. J Allergy Clin Immunol. 2018; 141:1590–1597.e9.
Article20. Chen H, Gould MK, Blanc PD, Miller DP, Kamath TV, Lee JH, et al. Asthma control, severity, and quality of life: quantifying the effect of uncontrolled disease. J Allergy Clin Immunol. 2007; 120:396–402.
Article21. Sullivan SD, Wenzel SE, Bresnahan BW, Zheng B, Lee JH, Pritchard M, et al. Association of control and risk of severe asthma-related events in severe or difficult-to-treat asthma patients. Allergy. 2007; 62:655–660.
Article22. Meltzer EO, Busse WW, Wenzel SE, Belozeroff V, Weng HH, Feng J, et al. Use of the Asthma Control Questionnaire to predict future risk of asthma exacerbation. J Allergy Clin Immunol. 2011; 127:167–172.
Article23. Bateman ED, Reddel HK, Eriksson G, Peterson S, Östlund O, Sears MR, et al. Overall asthma control: the relationship between current control and future risk. J Allergy Clin Immunol. 2010; 125:600–608. 608.e1–606.
Article24. Haselkorn T, Fish JE, Zeiger RS, Szefler SJ, Miller DP, Chipps BE, et al. Consistently very poorly controlled asthma, as defined by the impairment domain of the Expert Panel Report 3 guidelines, increases risk for future severe asthma exacerbations in The Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens (TENOR) study. J Allergy Clin Immunol. 2009; 124:895–902.e1-4.
Article25. Fuhlbrigge A, Peden D, Apter AJ, Boushey HA, Camargo CA Jr, Gern J, et al. Asthma outcomes: exacerbations. J Allergy Clin Immunol. 2012; 129:S34–48.
Article26. McDonald VM, Hiles SA, Godbout K, Harvey ES, Marks GB, Hew M, et al. Treatable traits can be identified in a severe asthma registry and predict future exacerbations. Respirology. 2019; 24:37–47.
Article27. Bel EH, Sousa A, Fleming L, Bush A, Chung KF, Versnel J, et al. Diagnosis and definition of severe refractory asthma: an international consensus statement from the Innovative Medicine Initiative (IMI). Thorax. 2011; 66:910–917.
Article28. Miller MK, Lee JH, Miller DP, Wenzel SE. TENOR Study Group. Recent asthma exacerbations: a key predictor of future exacerbations. Respir Med. 2007; 101:481–489.
Article29. Calhoun WJ, Haselkorn T, Mink DR, Miller DP, Dorenbaum A, Zeiger RS. Clinical burden and predictors of asthma exacerbations in patients on guideline-based steps 4–6 asthma therapy in the TENOR cohort. J Allergy Clin Immunol Pract. 2014; 2:193–200.
Article30. Tanaka A, Uno T, Sato H, Jinno M, Hirai K, Miyata Y, et al. Predicting future risk of exacerbations in Japanese patients with adult asthma: a prospective 1-year follow up study. Allergol Int. 2017; 66:568–573.
Article31. Kang HR, Song HJ, Nam JH, Hong SH, Yang SY, Ju S, et al. Risk factors of asthma exacerbation based on asthma severity: a nationwide population-based observational study in South Korea. BMJ Open. 2018; 8:e020825.
Article32. Boer S, Sont JK, Loijmans RJ, Snoeck-Stroband JB, ter Riet G, Schermer TR, et al. Development and validation of personalized prediction to estimate future risk of severe exacerbations and uncontrolled asthma in patients with asthma, using clinical parameters and early treatment response. J Allergy Clin Immunol Pract. 2019; 7:175–182.e5.
Article33. Bloom CI, Palmer T, Feary J, Quint JK, Cullinan P. Exacerbation patterns in adults with asthma in England. a population-based study. Am J Respir Crit Care Med. 2019; 199:446–453.
Article34. Patel M, Pilcher J, Reddel HK, Qi V, Mackey B, Tranquilino T, et al. Predictors of severe exacerbations, poor asthma control, and β-agonist overuse for patients with asthma. J Allergy Clin Immunol Pract. 2014; 2:751–758.
Article35. Loymans RJ, Honkoop PJ, Termeer EH, Snoeck-Stroband JB, Assendelft WJ, Schermer TR, et al. Identifying patients at risk for severe exacerbations of asthma: development and external validation of a multivariable prediction model. Thorax. 2016; 71:838–846.
Article36. Park HW, Song WJ, Kim SH, Park HK, Kim SH, Kwon YE, et al. Classification and implementation of asthma phenotypes in elderly patients. Ann Allergy Asthma Immunol. 2015; 114:18–22.
Article37. Bateman ED, Buhl R, O'Byrne PM, Humbert M, Reddel HK, Sears MR, et al. Development and validation of a novel risk score for asthma exacerbations: the risk score for exacerbations. J Allergy Clin Immunol. 2015; 135:1457–1464.e4.
Article38. Kupczyk M, ten Brinke A, Sterk PJ, Bel EH, Papi A, Chanez P, et al. Frequent exacerbators--a distinct phenotype of severe asthma. Clin Exp Allergy. 2014; 44:212–221.39. Song WJ, Sintobin I, Sohn KH, Kang MG, Park HK, Jo EJ, et al. Staphylococcal enterotoxin IgE sensitization in late-onset severe eosinophilic asthma in the elderly. Clin Exp Allergy. 2016; 46:411–421.
Article40. Bloom CI, Nissen F, Douglas IJ, Smeeth L, Cullinan P, Quint JK. Exacerbation risk and characterisation of the UK's asthma population from infants to old age. Thorax. 2018; 73:313–320.
Article41. Mukherjee M, Nair P. Autoimmune responses in severe asthma. Allergy Asthma Immunol Res. 2018; 10:428–447.
Article42. Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012; 380:651–659.
Article43. Ortega HG, Yancey SW, Mayer B, Gunsoy NB, Keene ON, Bleecker ER, et al. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respir Med. 2016; 4:549–556.
Article44. Hanania NA, Wenzel S, Rosén K, Hsieh HJ, Mosesova S, Choy DF, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013; 187:804–811.45. Wenzel S, Swanson B, Teper A, Hamilton J, Izuhara K, Ohta S, et al. Dupilumab reduces severe exacerbations in periostin-high and periostin-low asthma patients. Eur Respir J. 2016; 48:OA1798.
Article46. Newby C, Agbetile J, Hargadon B, Monteiro W, Green R, Pavord I, et al. Lung function decline and variable airway inflammatory pattern: longitudinal analysis of severe asthma. J Allergy Clin Immunol. 2014; 134:287–294.
Article47. American Thoracic Society. Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med. 2000; 162:2341–2351.48. Matsunaga K, Akamatsu K, Miyatake A, Ichinose M. Natural history and risk factors of obstructive changes over a 10-year period in severe asthma. Respir Med. 2013; 107:355–360.
Article49. Almeida PC, Ponte EV, Souza-Machado A, Cruz AA. Longitudinal trends in clinical characteristics and lung function of patients with severe asthma under treatment in Brazil. BMC Pulm Med. 2016; 16:141.
Article50. Fardet L, Petersen I, Nazareth I. Prevalence of long-term oral glucocorticoid prescriptions in the UK over the past 20 years. Rheumatology (Oxford). 2011; 50:1982–1990.
Article51. van Staa TP, Leufkens HG, Abenhaim L, Begaud B, Zhang B, Cooper C. Use of oral corticosteroids in the United Kingdom. QJM. 2000; 93:105–111.
Article52. Overman RA, Yeh JY, Deal CL. Prevalence of oral glucocorticoid usage in the United States: a general population perspective. Arthritis Care Res (Hoboken). 2013; 65:294–298.
Article53. Sarnes E, Crofford L, Watson M, Dennis G, Kan H, Bass D. Incidence and US costs of corticosteroid-associated adverse events: a systematic literature review. Clin Ther. 2011; 33:1413–1432.
Article54. Rice JB, White AG, Scarpati LM, Wan G, Nelson WW. Long-term systemic corticosteroid exposure: a systematic literature review. Clin Ther. 2017; 39:2216–2229.
Article55. Lefebvre P, Duh MS, Lafeuille MH, Gozalo L, Desai U, Robitaille MN, et al. Acute and chronic systemic corticosteroid-related complications in patients with severe asthma. J Allergy Clin Immunol. 2015; 136:1488–1495.
Article56. Dalal AA, Duh MS, Gozalo L, Robitaille MN, Albers F, Yancey S, et al. Dose-response relationship between long-term systemic corticosteroid use and related complications in patients with severe asthma. J Manag Care Spec Pharm. 2016; 22:833–847.
Article57. Sullivan PW, Ghushchyan VH, Globe G, Schatz M. Oral corticosteroid exposure and adverse effects in asthmatic patients. J Allergy Clin Immunol. 2018; 141:110–116.e7.
Article58. Sweeney J, Patterson CC, Menzies-Gow A, Niven RM, Mansur AH, Bucknall C, et al. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the Optimum Patient Care Research Database and the British Thoracic Difficult Asthma Registry. Thorax. 2016; 71:339–346.
Article59. Barry LE, O'Neill C, Patterson C, Sweeney J, Price D, Heaney LG. Age and sex associations with systemic corticosteroid-induced morbidity in asthma. J Allergy Clin Immunol Pract. 2018; 6:2014–2023.e2.
Article60. Bloechliger M, Reinau D, Spoendlin J, Chang SC, Kuhlbusch K, Heaney LG, et al. Adverse events profile of oral corticosteroids among asthma patients in the UK: cohort study with a nested case-control analysis. Respir Res. 2018; 19:75.
Article61. Daugherty J, Lin X, Baxter R, Suruki R, Bradford E. The impact of long-term systemic glucocorticoid use in severe asthma: a UK retrospective cohort analysis. J Asthma. 2018; 55:651–658.
Article62. Price DB, Trudo F, Voorham J, Xu X, Kerkhof M, Ling Zhi Jie J, et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J Asthma Allergy. 2018; 11:193–204.
Article63. Heffler E, Madeira LN, Ferrando M, Puggioni F, Racca F, Malvezzi L, et al. Inhaled corticosteroids safety and adverse effects in patients with asthma. J Allergy Clin Immunol Pract. 2018; 6:776–781.
Article64. Wong CA, Walsh LJ, Smith CJ, Wisniewski AF, Lewis SA, Hubbard R, et al. Inhaled corticosteroid use and bone-mineral density in patients with asthma. Lancet. 2000; 355:1399–1403.
Article65. Choi YJ, Lee HY, Yoon D, Kim A, Shin YS, Park HS, et al. Trabecular bone score is more sensitive to asthma severity and glucocorticoid treatment than bone mineral density in asthmatics. Allergy Asthma Immunol Res. 2019; 11:343–356.
Article66. Weatherall M, James K, Clay J, Perrin K, Masoli M, Wijesinghe M, et al. Dose-response relationship for risk of non-vertebral fracture with inhaled corticosteroids. Clin Exp Allergy. 2008; 38:1451–1458.
Article67. Garbe E, Suissa S, LeLorier J. Association of inhaled corticosteroid use with cataract extraction in elderly patients. JAMA. 1998; 280:539–543.
Article68. Bourdin A, Molinari N, Vachier I, Pahus L, Suehs C, Chanez P. Mortality: a neglected outcome in OCS-treated severe asthma. Eur Respir J. 2017; 50:1701486.69. Bourdin A, Fabry-Vendrand C, Ostinelli J, Ait-Yahia M, Darnal E, Bouee S, et al. The burden of severe asthma in France: a case-control study using a medical claims database. J Allergy Clin Immunol Pract. 2019; 7:1477–1487.
Article70. Fernandes AG, Souza-Machado C, Coelho RC, Franco PA, Esquivel RM, Souza-Machado A, et al. Risk factors for death in patients with severe asthma. J Bras Pneumol. 2014; 40:364–372.
Article71. Fajt ML, Wenzel SE. Development of new therapies for severe asthma. Allergy Asthma Immunol Res. 2017; 9:3–14.
Article72. Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014; 371:1189–1197.
Article73. Nair P, Wenzel S, Rabe KF, Bourdin A, Lugogo NL, Kuna P, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med. 2017; 376:2448–2458.
Article74. Rabe KF, Nair P, Brusselle G, Maspero JF, Castro M, Sher L, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. 2018; 378:2475–2485.
Article75. Khatri S, Moore W, Gibson PG, Leigh R, Bourdin A, Maspero J, et al. Assessment of the long-term safety of mepolizumab and durability of clinical response in patients with severe eosinophilic asthma. J Allergy Clin Immunol. 2019; 143:1742–1751.e7.
Article76. Chung KF, Adcock IM. Precision medicine for the discovery of treatable mechanisms in severe asthma. Allergy. 2019.
Article77. Zazzali JL, Broder MS, Omachi TA, Chang E, Sun GH, Raimundo K. Risk of corticosteroid-related adverse events in asthma patients with high oral corticosteroid use. Allergy Asthma Proc. 2015; 36:268–274.
Article78. Lefebvre P, Duh MS, Lafeuille MH, Gozalo L, Desai U, Robitaille MN, et al. Burden of systemic glucocorticoid-related complications in severe asthma. Curr Med Res Opin. 2017; 33:57–65.
Article