Tuberc Respir Dis.
2019 Oct;82(4):306-310. 10.4046/trd.2019.0034.
Experiences of Latent Tuberculosis Infection Treatment for the North Korean Refugees
- Affiliations
-
- 1Division of Pulmonary, Sleep and Critical Care Medicine, Department of Internal Medicine, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea. lee-sh@korea.ac.kr
- 2Korea Institute of Tuberculosis, Cheongju, Korea.
- 3Medical Science Research Center, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea.
- 4Settlement Support Center for North Korean Refugees (Hanawon), Hwacheon, Korea.
- KMID: 2459054
- DOI: http://doi.org/10.4046/trd.2019.0034
Abstract
- BACKGROUND
Tuberculosis (TB) is increasing in immigrants. We aimed to investigate the current status of latent tuberculosis infection (LTBI) treatment for North Korean Refugees (NKR) compared to South Koreans Contacts (SKC).
METHODS
TB close contacts in a closed facility of SKC and NKR who underwent LTBI screening in a settlement support center for NKR were analyzed retrospectively.
RESULTS
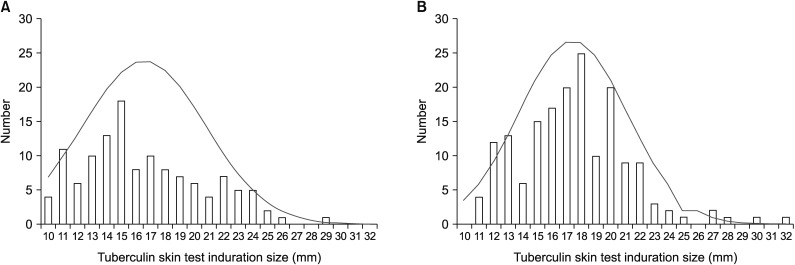
Among tuberculin skin test (TST) ≥10 mm (n=298) reactors, the males accounted for 72.2% in SKC (n=126) and 19.5% in NKR (n=172) (p<0.01). The mean age was higher in South Korea (42.8±9.9 years vs. 35.4±10.0 years, p<0.01). Additionally, the mean TST size was significantly bigger in NKR (17.39±3.9 mm vs. 16.57±4.2 mm, p=0.03). The LTBI treatments were initiated for all screened NKR, and LTBI completion rate was only 68.0%. However, in NKR, LTBI treatment completion rate was significantly increased by shorter 4R regimen (odds ratio [OR], 9.296; 95% confidence interval [CI], 4.159-20.774; p<0.01) and male (OR, 3.447; 95% CI, 1.191-9.974; p=0.02).
CONCLUSION
LTBI treatment compliance must be improved in NKR with a shorter regimen. In addition, a larger study regarding a focus on LTBI with easy access to related data for NKR should be conducted.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
One Step toward a Low Tuberculosis-Burden Country: Screening for Tuberculosis Infection among the Immigrants and Refugees
Hyung Woo Kim, Ju Sang Kim
Tuberc Respir Dis. 2020;83(1):104-105. doi: 10.4046/trd.2019.0079.
Reference
-
1. Korea Centers for Disease Control and Prevention. Annual report on the notified tuberculosis in Korea, 2018 [Internet]. Cheongju: Korea Centers for Disease Control and Prevention;2019. cited 2019 Apr 30. Available from: http://tbzero.cdc.go.kr/tbzero/board/boardView.do?leftMenuId=48¶mMenuId=77&boardSeq=5464&crudType=R.2. World Health Organization. Implementing the end TB strategy: the essentials. WHO/HTM/TB [Internet]. Geneva: World Health Organization;2015. cited 2019 Apr 30. Available from: https://www.who.int/tb/publications/2015/The_Essentials_to_End_TB/en/.3. Cho KS. Tuberculosis control in the Republic of Korea. Epidemiol Health. 2018; 40:e2018036. PMID: 30081621.
Article4. Min GH, Kim Y, Lee JS, Oh JY, Hur GY, Lee YS, et al. Social and clinical characteristics of immigrants with tuberculosis in South Korea. Yonsei Med J. 2017; 58:592–597. PMID: 28332365.
Article5. Lee S, Ryu JY, Kim DH. Pre-immigration screening for tuberculosis in South Korea: a comparison of smear- and culture-based protocols. Tuberc Respir Dis. 2019; 82:151–157.
Article6. Choi CM, June JH, Kang CI, Park JT, Oh SY, Lee JB, et al. Tuberculosis among dislocated North Koreans entering Republic of Korea since 1999. J Korean Med Sci. 2007; 22:963–967. PMID: 18162707.
Article7. Ann SY, Ryou SH, Kim SB. Clinical characteristics of defectors from North Korea visiting a single tertiary hospital in South Korea. Korean J Med. 2015; 89:54–63.
Article8. Farhat M, Greenaway C, Pai M, Menzies D. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria. Int J Tuberc Lung Dis. 2006; 10:1192–1204. PMID: 17131776.9. Joint Committee for the Revision of Korean Guidelines for Tuberculosis. Korean Centers for Disease Control and Prevention. Korean guidelines for tuberculosis. 3rd ed. Cheonju: Korea Centers for Disease Control and Prevention;2017.10. Fox GJ, Dobler CC, Marais BJ, Denholm JT. Preventive therapy for latent tuberculosis infection-the promise and the challenges. Int J Infect Dis. 2017; 56:68–76. PMID: 27872018.
Article11. Chen S, Zhang H, Pan Y, Long Q, Xiang L, Yao L, et al. Are free anti-tuberculosis drugs enough? An empirical study from three cities in China. Infect Dis Poverty. 2015; 4:47. PMID: 26510711.
Article12. Venkatesan P. Changing the treatment landscape for latent tuberculosis with rifampicin. Lancet Respir Med. 2018; 6:740. PMID: 30104169.
Article13. Menzies D, Adjobimey M, Ruslami R, Trajman A, Sow O, Kim H, et al. Four months of rifampin or nine months of isoniazid for latent tuberculosis in adults. N Engl J Med. 2018; 379:440–453. PMID: 30067931.
Article14. Lee SH, Lew WJ, Kim HJ, Lee HK, Lee YM, Cho CH, et al. Serial interferon-gamma release assays after rifampicin prophylaxis in a tuberculosis outbreak. Respir Med. 2010; 104:448–453. PMID: 19879123.
Article15. Lee SH, Yim JJ, Kim HJ, Shim TS, Seo HS, Cho YS, et al. Adverse events and development of tuberculosis after 4 months of rifampicin prophylaxis in a tuberculosis outbreak. Epidemiol Infect. 2012; 140:1028–1035. PMID: 21835069.
Article16. Diel R, Loddenkemper R, Meywald-Walter K, Gottschalk R, Nienhaus A. Comparative performance of tuberculin skin test, QuantiFERON-TB-Gold In Tube assay, and T-Spot.TB test in contact investigations for tuberculosis. Chest. 2009; 135:1010–1018. PMID: 19017873.17. Seung KJ, Linton SW. The growing problem of multidrugresistant tuberculosis in North Korea. PLoS Med. 2013; 10:e1001486. PMID: 23935457.
Article18. Kim YN. Narrative Inquiry about entering process South Korea, NIS investigation course and Hanawon center curriculum of North Korean defectors. J Hum Rights Law Relat Educ. 2016; 9:33–64.19. Park SJ, Jo KW, Yoo B, Lee CK, Kim YG, Yang SK, et al. Comparison of LTBI treatment regimens for patients receiving anti-tumour necrosis factor therapy. Int J Tuberc Lung Dis. 2015; 19:342–348. PMID: 25686145.
Article20. Assefa Y, Assefa Y, Woldeyohannes S, Hamada Y, Getahun H. 3-month daily rifampicin and isoniazid compared to 6- or 9-month isoniazid for treating latent tuberculosis infection in children and adolescents less than 15 years of age: an updated systematic review. Eur Respir J. 2018; 52:1800395. PMID: 29748305.
Article21. Park SM, Lee HW. Current status of healthcare and effective health aid strategies in North Korea. J Korean Med Assoc. 2013; 56:368–374.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Psychiatric Symptoms on Attention in North Korean Refugees
- Clinical Characteristics of Tuberculosis in North Korean Refugees
- Lived Experience of Psychological Suffering among the North Korean Refugees: Applied to Parse's Human Becoming Research Methodology
- Traumatic Experiences and Mental Health of North Korean Refugees in South Korea
- Difference of Traumatic Experience according to Menstrual Regularity among North Korean Woman Refugees in South Korea