Angled Cool-Tip Electrode for Radiofrequency Ablation of Small Superficial Subcapsular Tumors in the Liver: A Feasibility Study
- Affiliations
-
- 1Department of Radiology, Research Institute of Radiological Science, Severance Hospital, Yonsei University College of Medicine, Seoul 03722, Korea. astarte@yuhs.ac
- 2Department of Radiology, Bucheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Bucheon 14647, Korea.
- 3Department of Radiology, CHA Bundang Medical Center, CHA University, Seongnam 13496, Korea.
- 4Department of Surgery, Severance Hospital, Yonsei University College of Medicine, Seoul 03722, Korea.
- 5Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul 03722, Korea.
- KMID: 2458066
- DOI: http://doi.org/10.3348/kjr.2016.17.5.742
Abstract
OBJECTIVE
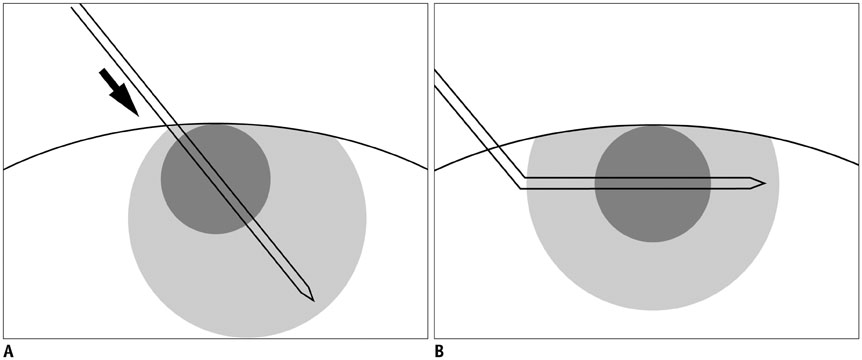
To evaluate the feasibility of angled cool-tip electrode for radiofrequency ablation of small superficial subcapsular liver tumors abutting abdominal wall, in order to traverse normal liver parenchyma, and thereby, obtain favorable configuration of ablation margin.
MATERIALS AND METHODS
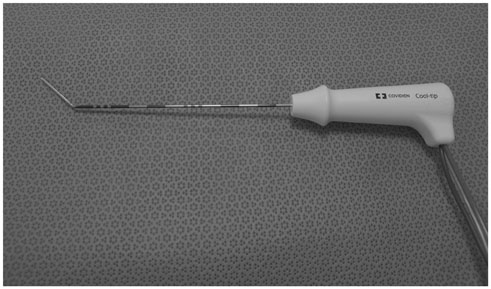
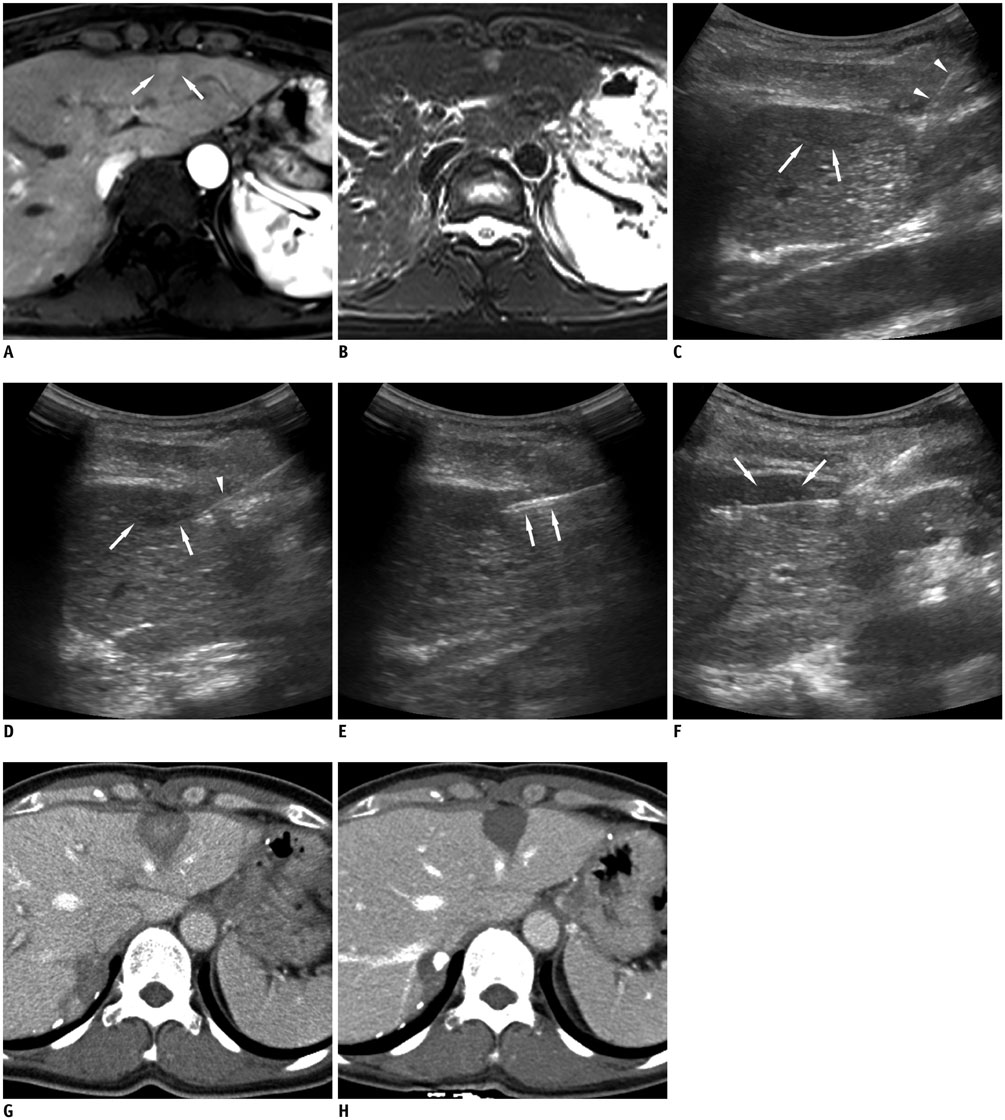
In this study, we retrospectively analyzed 15 small superficial subcapsular liver tumors abutting abdominal wall in 15 patients, treated with radiofrequency ablation from March 2013 to June 2015 using a cool-tip electrode manually modified to create 25-35° angle at the junction between exposed and insulated segments. The tumors were hepatocellular carcinoma (n = 13) and metastases (n = 2: cholangiocellular carcinoma and rectosigmoid cancer), with maximum diameter of 10-26 mm (mean, 15.68 ± 5.29 mm). Under ultrasonographic guidance, the electrode tip was advanced to the depth of the tumors' epicenter about 1 cm from the margin. The tip was re-directed to penetrate the tumor for radiofrequency ablation. Minimal ablation margin was measured at immediate post-treatment CT. Radiological images and medical records were evaluated for success rate, length of minimal ablation margin and complications.
RESULTS
Technical success rate of obtaining complete necrosis of the tumors was 100%, with no procedure-related complication. Minimal ablation margin ranged from 3-12 mm (mean, 7.07 ± 2.23 mm). CT/MRI follow-up at 21-1022 days (mean, 519.47 ± 304.51 days) revealed no local recurrence, but distant recurrence in 9 patients.
CONCLUSION
Using an angled cool-tip electrode for radiofrequency ablation of small superficial subcapsular tumors abutting abdominal wall may be a feasible technique for obtaining adequate ablation margin and lower complication rate.
Keyword
MeSH Terms
-
Adult
Aged
Carcinoma, Hepatocellular/pathology/secondary/*surgery
Catheter Ablation/adverse effects/*instrumentation/methods
Electrodes
Equipment Design
Feasibility Studies
Female
Humans
Liver Neoplasms/pathology/*surgery
Magnetic Resonance Imaging
Male
Middle Aged
Neoplasm Recurrence, Local/surgery
Retrospective Studies
Tomography, X-Ray Computed
Figure
Cited by 3 articles
-
No-Touch Radiofrequency Ablation of VX2 Hepatic Tumors
In Vivo in Rabbits: A Proof of Concept Study
Tae-Hyung Kim, Hyoung In Choi, Bo Ram Kim, Ji Hee Kang, Ju Gang Nam, Sae Jin Park, Seunghyun Lee, Jeong Hee Yoon, Dong Ho Lee, Ijin Joo, Jeong Min Lee
Korean J Radiol. 2018;19(6):1099-1109. doi: 10.3348/kjr.2018.19.6.1099.Ultrasound-Guided Intraoperative Radiofrequency Ablation and Surgical Resection for Liver Metastasis from Malignant Gastrointestinal Stromal Tumors
In Sun Yoon, Ji Hoon Shin, Kichang Han, Pyo Nyun Kim, Ki Hun Kim, Yoon-Koo Kang, Heung Kyu Ko
Korean J Radiol. 2018;19(1):54-62. doi: 10.3348/kjr.2018.19.1.54.Impact of Energy and Access Methods on Extrahepatic Tumor Spreading and the Ablation Zone: An
Ex vivo Experiment Using a Subcapsular Tumor Model
Jin Sil Kim, Youngsun Ko, Hyeyoung Kwon, Minjeong Kim, Jeong Kyong Lee
Korean J Radiol. 2019;20(4):580-588. doi: 10.3348/kjr.2018.0564.
Reference
-
1. Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012; 107:569–577. quiz 578.2. Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999; 210:655–661.3. Solbiati L, Livraghi T, Goldberg SN, Ierace T, Meloni F, Dellanoce M, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001; 221:159–166.4. Yoon JH, Lee JM, Hwang EJ, Hwang IP, Baek J, Han JK, et al. Monopolar radiofrequency ablation using a dual-switching system and a separable clustered electrode: evaluation of the in vivo efficiency. Korean J Radiol. 2014; 15:235–244.5. Rossi S, Buscarini E, Garbagnati F, Di Stasi M, Quaretti P, Rago M, et al. Percutaneous treatment of small hepatic tumors by an expandable RF needle electrode. AJR Am J Roentgenol. 1998; 170:1015–1022.6. Pompili M, Saviano A, de Matthaeis N, Cucchetti A, Ardito F, Federico B, et al. Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma ≤3 cm. Results of a multicenter Italian survey. J Hepatol. 2013; 59:89–97.7. Cho YK, Kim JK, Kim WT, Chung JW. Hepatic resection versus radiofrequency ablation for very early stage hepatocellular carcinoma: a Markov model analysis. Hepatology. 2010; 51:1284–1290.8. de Baère T, Risse O, Kuoch V, Dromain C, Sengel C, Smayra T, et al. Adverse events during radiofrequency treatment of 582 hepatic tumors. AJR Am J Roentgenol. 2003; 181:695–700.9. Mulier S, Mulier P, Ni Y, Miao Y, Dupas B, Marchal G, et al. Complications of radiofrequency coagulation of liver tumours. Br J Surg. 2002; 89:1206–1222.10. Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003; 226:441–451.11. Rhim H, Yoon KH, Lee JM, Cho Y, Cho JS, Kim SH, et al. Major complications after radio-frequency thermal ablation of hepatic tumors: spectrum of imaging findings. Radiographics. 2003; 23:123–134. discussion 134-136.12. Teratani T, Yoshida H, Shiina S, Obi S, Sato S, Tateishi R, et al. Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology. 2006; 43:1101–1108.13. Kim YS, Rhim H, Cho OK, Koh BH, Kim Y. Intrahepatic recurrence after percutaneous radiofrequency ablation of hepatocellular carcinoma: analysis of the pattern and risk factors. Eur J Radiol. 2006; 59:432–441.14. Yang W, Yan K, Wu GX, Wu W, Fu Y, Lee JC, et al. Radiofrequency ablation of hepatocellular carcinoma in difficult locations: strategies and long-term outcomes. World J Gastroenterol. 2015; 21:1554–1566.15. Llovet JM, Vilana R, Brú C, Bianchi L, Salmeron JM, Boix L, et al. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001; 33:1124–1129.16. Shirai K, Tamai H, Shingaki N, Mori Y, Moribata K, Enomoto S, et al. Clinical features and risk factors of extrahepatic seeding after percutaneous radiofrequency ablation for hepatocellular carcinoma. Hepatol Res. 2011; 41:738–745.17. Jaskolka JD, Asch MR, Kachura JR, Ho CS, Ossip M, Wong F, et al. Needle tract seeding after radiofrequency ablation of hepatic tumors. J Vasc Interv Radiol. 2005; 16:485–491.18. Imamura J, Tateishi R, Shiina S, Goto E, Sato T, Ohki T, et al. Neoplastic seeding after radiofrequency ablation for hepatocellular carcinoma. Am J Gastroenterol. 2008; 103:3057–3062.19. Livraghi T, Lazzaroni S, Meloni F, Solbiati L. Risk of tumour seeding after percutaneous radiofrequency ablation for hepatocellular carcinoma. Br J Surg. 2005; 92:856–858.20. Sartori S, Tombesi P, Macario F, Nielsen I, Tassinari D, Catellani M, et al. Subcapsular liver tumors treated with percutaneous radiofrequency ablation: a prospective comparison with nonsubcapsular liver tumors for safety and effectiveness. Radiology. 2008; 248:670–679.21. Poon RT, Ng KK, Lam CM, Ai V, Yuen J, Fan ST. Radiofrequency ablation for subcapsular hepatocellular carcinoma. Ann Surg Oncol. 2004; 11:281–289.22. Crocetti L, de Baere T, Lencioni R. Quality improvement guidelines for radiofrequency ablation of liver tumours. Cardiovasc Intervent Radiol. 2010; 33:11–17.23. Patel PA, Ingram L, Wilson ID, Breen DJ. No-touch wedge ablation technique of microwave ablation for the treatment of subcapsular tumors in the liver. J Vasc Interv Radiol. 2013; 24:1257–1262.24. Korean Liver Cancer Study Group (KLCSG). National Cancer Center, Korea (NCC). 2014 Korean Liver Cancer Study Group-National Cancer Center Korea practice guideline for the management of hepatocellular carcinoma. Korean J Radiol. 2015; 16:465–522.25. Yoon JH, Park JW, Lee JM. Noninvasive diagnosis of hepatocellular carcinoma: elaboration on Korean Liver Cancer Study Group-National Cancer Center Korea practice guidelines compared with other guidelines and remaining issues. Korean J Radiol. 2016; 17:7–24.26. Wang X, Sofocleous CT, Erinjeri JP, Petre EN, Gonen M, Do KG, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013; 36:166–175.27. Kim YS, Lee WJ, Rhim H, Lim HK, Choi D, Lee JY. The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (> 2 and < 5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. AJR Am J Roentgenol. 2010; 195:758–765.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Radiofrequency Thermal Ablation of Hepatocellular Carcinomas
- Feasibility of Saline Infusion on the Liver Surface during Radiofrequency Ablation of Subcapsular Hepatic Tumor: An Experimental Study
- Radiofrequency Tissue Ablation with Cooled-Tip Electrodes:An Experimental Study in a Bovine Liver Model on Variables Influencing Lesion Size
- Impact of Energy and Access Methods on Extrahepatic Tumor Spreading and the Ablation Zone: An Ex vivo Experiment Using a Subcapsular Tumor Model
- Does Artificial Ascites Induce the Heat-Sink Phenomenon during Percutaneous Radiofrequency Ablation of the Hepatic Subcapsular Area?: an in vivo Experimental Study Using a Rabbit Model