Korean J Radiol.
2009 Feb;10(1):43-50. 10.3348/kjr.2009.10.1.43.
Does Artificial Ascites Induce the Heat-Sink Phenomenon during Percutaneous Radiofrequency Ablation of the Hepatic Subcapsular Area?: an in vivo Experimental Study Using a Rabbit Model
- Affiliations
-
- 1Department of Radiology and Center for Imaging Science, Samsung Medical Center, Sunkyunkwan University School of Medicine, Seoul, Korea. rhimhc@skku.edu
- KMID: 1088677
- DOI: http://doi.org/10.3348/kjr.2009.10.1.43
Abstract
OBJECTIVE
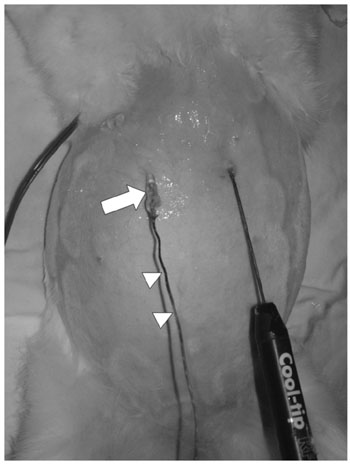
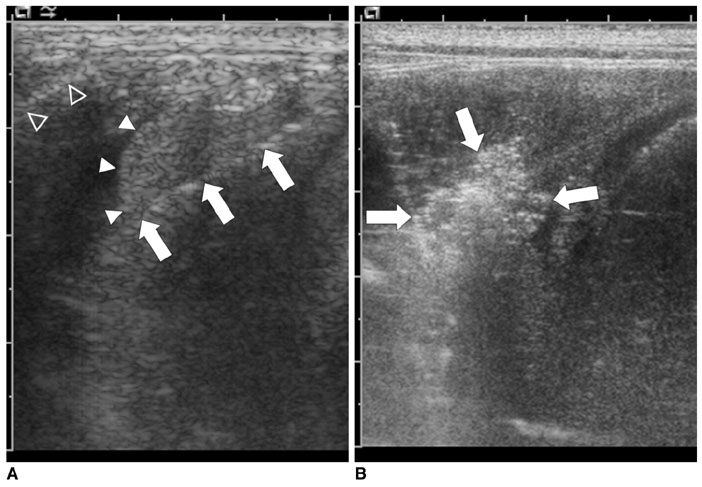
To evaluate the effect of the heat-sink phenomenon induced by artificial ascites on the size of the ablation zone during percutaneous radiofrequency (RF) ablation of the hepatic subcapsular area in an in vivo rabbit model. MATERIALS AND METHODS: A total of 21 percutaneous rabbit liver RF ablations were performed with and without artificial ascites (5% dextrose aqueous solution). The rabbits were divided into three groups: a) control group (C, n = 7); b) room temperature ascites group (R, n = 7); and c) warmed ascites group (W, n = 7). The tip of a 1 cm, internally cooled electrode was placed on the subcapsular region of the hepatic dome via ultrasound guidance, and ablation was continued for 6 min. Changes in temperature of the ascites were monitored during the ablation. The size of the ablation zones of the excised livers and immediate complications rates were compared statistically between the groups (Mann-Whitney U test, Kruskal-Wallis test, linear-by-linear association, p = 0.05). RESULTS: One rabbit from the "W" group expired during the procedure. In all groups, the ascites temperatures approached their respective body temperatures as the ablations continued; however, a significant difference in ascites temperature was found between groups "W" and "R" throughout the procedures (39.2 +/- 0.4 degrees C in group W and 33.4 +/- 4.3 degrees C in group R at 6 min, p = 0.003). No significant difference was found between the size of the ablation zones (782.4 +/- 237.3 mL in group C, 1,172.0 +/- 468.9 mL in group R, and 1,030.6 +/- 665.1 mL in group W, p = 0.170) for the excised liver specimens. Diaphragmatic injury was identified in three of seven cases (42.9%) upon visual inspection of group "C" rabbits (p = 0.030). CONCLUSION: Artificial ascites are not likely to cause a significant heat-sink phenomenon in the percutaneous RF ablation of the hepatic subcapsular region.
Keyword
MeSH Terms
Figure
Reference
-
1. Choi D, Lim HK, Rhim H, Kim YS, Yoo BC, Paik SW, et al. Percutaneous radiofrequency ablation for recurrent hepatocellular carcinoma after hepatectomy: long-term results and prognostic factors. Ann Surg Oncol. 2007. 14:2319–2329.2. Choi D, Lim HK, Rhim H, Kim YS, Lee WJ, Paik SW, et al. Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first-line treatment: long-term results and prognostic factors in a large single-institution series. Eur Radiol. 2007. 17:684–692.3. Hong SN, Lee SY, Choi MS, Lee JH, Koh KC, Paik SW, et al. Comparing the outcomes of radiofrequency ablation and surgery in patients with a single small hepatocellular carcinoma and well-preserved hepatic function. J Clin Gastroenterol. 2005. 39:247–252.4. Kim YS, Rhim H, Paik SS. Radiofrequency ablation of the liver in a rabbit model: creation of artificial ascites to minimize collateral thermal injury to the diaphragm and stomach. J Vasc Interv Radiol. 2006. 17:541–547.5. Hinshaw JL, Laeseke PF, Winter TC 3rd, Kliewer MA, Fine JP, Lee FT Jr. Radiofrequency ablation of peripheral liver tumors: intraperitoneal 5% dextrose in water decreases postprocedural pain. AJR Am J Roentgenol. 2006. 186:S306–S310.6. Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation with artificial ascites for hepatocellular carcinoma in the hepatic dome: initial experience. AJR Am J Roentgenol. 2008. 190:91–98.7. Lu DS, Raman SS, Vodopich DJ, Wang M, Sayre J, Lassman C. Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: assessment of the "heat sink" effect. AJR Am J Roentgenol. 2002. 178:47–51.8. Kim SK, Lim HK, Ryu JA, Choi D, Lee WJ, Lee JY, et al. Radiofrequency ablation of rabbit liver in vivo: effect of the pringle maneuver on pathologic changes in liver surrounding the ablation zone. Korean J Radiol. 2004. 5:240–249.9. Kapoor BS, Hunter DW. Injection of subphrenic saline during radiofrequency ablation to minimize diaphragmatic injury. Cardiovasc Intervent Radiol. 2003. 26:302–304.10. Laeseke PF, Sampson LA, Brace CL, Winter TC 3rd, Fine JP, Lee FT Jr. Unintended thermal injuries from radiofrequency ablation: protection with 5% dextrose in water. AJR Am J Roentgenol. 2006. 186:S249–S254.11. Ohmoto K, Yamamoto S. Percutaneous microwave coagulation therapy using artificial ascites. AJR Am J Roentgenol. 2001. 176:817–818.12. Ohmoto K, Tsuzuki M, Yamamoto S. Percutaneous microwave coagulation therapy with intraperitoneal saline infusion for hepatocellular carcinoma in the hepatic dome. AJR Am J Roentgenol. 1999. 172:65–66.13. Minami Y, Kudo M, Kawasaki T, Chung H, Ogawa C, Inoue T, et al. Percutaneous ultrasound-guided radiofrequency ablation with artificial pleural effusion for hepatocellular carcinoma in the hepatic dome. J Gastroenterol. 2003. 38:1066–1070.14. Shibata T, Iimuro Y, Ikai I, Hatano E, Yamaoka Y, Konishi J. Percutaneous radiofrequency ablation therapy after intrathoracic saline solution infusion for liver tumor in the hepatic dome. J Vasc Interv Radiol. 2002. 13:313–315.15. Lee YR, Rhim H, Kim YS, Cho OK, Koh BH, Kim Y, et al. Intraperitoneal saline infusion during radiofrequency ablation of subcapsular hepatic tumor. J Vasc Interv Radiol. 2005. 16:753–754.16. Raman SS, Lu DS, Vodopich DJ, Sayre J, Lassman C. Minimizing diaphragmatic injury during radio-frequency ablation: efficacy of subphrenic peritoneal saline injection in a porcine model. Radiology. 2002. 222:819–823.17. Kim YS, Rhim H, Lim HK, Park CK, Lee WJ, Do YS, et al. Completeness of treatment in hepatocellular carcinomas treated with image-guided tumor therapies: evaluation of positive predictive value of contrast-enhanced CT with histopathologic correlation in the explanted liver specimen. J Comput Assist Tomogr. 2006. 30:578–582.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Impact of Energy and Access Methods on Extrahepatic Tumor Spreading and the Ablation Zone: An Ex vivo Experiment Using a Subcapsular Tumor Model
- Feasibility of Saline Infusion on the Liver Surface during Radiofrequency Ablation of Subcapsular Hepatic Tumor: An Experimental Study
- Radiofrequency Ablation of Hepatic Cysts: Case Report
- Ultrasound-Guided Percutaneous Radiofrequency Ablation of Liver Tumors: How We Do It Safely and Completely
- Radiofrequency Ablation of Hepatocellular Carcinoma: Pros and Cons