Urogenit Tract Infect.
2019 Aug;14(2):64-70. 10.14777/uti.2019.14.2.64.
Korean Translation of the GRADE Series Published in the BMJ, ‘GRADE: What Is “Quality of Evidence†and Why Is It Important to Clinicians?’ (A Secondary Publication)
- Affiliations
-
- 1Department of Urology, Chungbuk National University Hospital, Chungbuk National University College of Medicine, Cheongju, Korea.
- 2Department of Urology, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 3Institute of Evidence Based Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 4Department of Urology, Soonchunhyang University Seoul Hospital, Soonchunhyang University College of Medicine, Seoul, Korea.
- 5Department of Urology, Pusan National University Hospital, Busan, Korea.
- 6Department of Urology, Catholic University of Daegu School of Medicine, Daegu, Korea.
- 7Department of Urology, College of Medicine, Konyang University, Daejeon, Korea.
- 8Department of Urology, Chonnam National University Medical School, Hwasun, Korea. urohwang@gmail.com
- KMID: 2456612
- DOI: http://doi.org/10.14777/uti.2019.14.2.64
Abstract
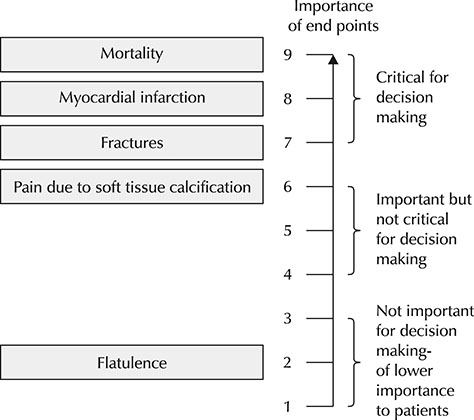
- This article is second translation of a GRADE series published in the BMJ to create a highly structured, transparent, and informative system for rating quality of evidence for developing recommendations. The process to develop a guideline, we should formulate a clear question with specification of all outcomes of importance to patients. Grading of Recommendations, Assessment, Development and Evaluation (GRADE) offers four levels of evidence quality: high, moderate, low, and very low for these patient-important outcomes. Randomized trials begin as high quality evidence and observational studies as low quality evidence. Although randomized trials begin as high quality evidence, quality may be downgraded as a result of study limitations (risk of bias), inconsistency (variability in results), indirectness, imprecision (wide confidence intervals), or publication bias. While the quality of evidence derived from observational studies starts at "˜low' but may be upgraded based on a very large magnitude of effect, a dose-response gradient, and if all plausible biases would reduce an apparent treatment effect.
MeSH Terms
Figure
Reference
-
1. Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004; 328:1490.
Article2. Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schunemann HJ. GRADE Working Group. What is “quality of evidence” and why is it important to clinicians? BMJ. 2008; 336:995–998.3. Oxman AD, Guyatt GH. Guidelines for reading literature reviews. CMAJ. 1988; 138:697–703.4. Schunemann HJ, Fretheim A, Oxman AD. Improving the use of research evidence in guideline development: 10. integrating values and consumer involvement. Health Res Policy Syst 2006;4:22.5. Fletcher SW, Spitzer WO. Approach of the Canadian task force to the periodic health examination. Ann Intern Med. 1980; 92:253–254.
Article6. Schunemann HJ, Jaeschke R, Cook DJ, Bria WF, El-Solh AA, Ernst A, et al. ATS Documents Development and Implementation Committee. An official ATS statement: grading the quality of evidence and strength of recommendations in ATS guidelines and recommendations. Am J Respir Crit Care Med. 2006; 174:605–614.7. Guyatt G, Cook D, Devereaux PJ, Meade M, Straus S. Therapy. In: Guyatt G, Rennie D, editors. Users\' guides to the medical literature: a manual for evidence-based clinical practice. Chicago: American Medical Association; 2002.8. Montori VM, Devereaux PJ, Adhikari NK, Burns KE, Eggert CH, Briel M, et al. Randomized trials stopped early for benefit: a systematic review. JAMA. 2005; 294:2203–2209.9. Karanicolas PJ, Davies E, Kunz R, Briel M, Koka HP, Payne DM, et al. The pylorus: take it or leave it? Systematic review and meta-analysis of pylorus-preserving versus standard whipple pancreaticoduodenectomy for pancreatic or periampullary cancer. Ann Surg Oncol. 2007; 14:1825–1834.
Article10. Glasziou P, Chalmers I, Rawlins M, McCulloch P. When are randomised trials unnecessary? Picking signal from noise. BMJ. 2007; 334:349–351.
Article11. Thompson DC, Rivara FP, Thompson R. Helmets for preventing head and facial injuries in bicyclists. Cochrane Database Syst Rev 2000;(2):CD001855.12. Cannegieter SC, Rosendaal FR, Briet E. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation. 1994; 89:635–641.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Korean Translation of the GRADE Series Published in the BMJ, ‘GRADE: Going from Evidence to Recommendations’ (A Secondary Publication)
- Korean Translation of the GRADE Series Published in the BMJ, ‘GRADE: Incorporating Considerations of Resources Use into Grading Recommendations’ (A Secondary Publication)
- Korean Translation of the GRADE Series Published in the BMJ, ‘GRADE: An Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations’ (A Secondary Publication)
- Korean Translation of the GRADE Series Published in the BMJ, ‘GRADE: Grading Quality of Evidence and Strength of Recommendations for Diagnostic Tests and Strategies’ (A Secondary Publication)
- Korean Translation of the GRADE Series Published in the BMJ, ‘Use of GRADE Grid to Reach Decisions on Clinical Practice Guidelines When Consensus Is Elusive’ (A Secondary Publication)