Investig Clin Urol.
2019 Sep;60(5):359-366. 10.4111/icu.2019.60.5.359.
Treatment with low-energy shock wave alleviates pain in an animal model of uroplakin 3A-induced autoimmune interstitial cystitis/painful bladder syndrome
- Affiliations
-
- 1Department of Urology, Peking University First Hospital, Peking University, Beijing, China. yingluguo@aliyun.com
- KMID: 2455963
- DOI: http://doi.org/10.4111/icu.2019.60.5.359
Abstract
- PURPOSE
To investigate whether treatment with low-energy shock wave (LESW) alleviates pain and bladder dysfunction in a mouse model of uroplakin 3A (UPK3A)-induced interstitial cystitis/painful bladder syndrome (IC/PBS).
MATERIALS AND METHODS
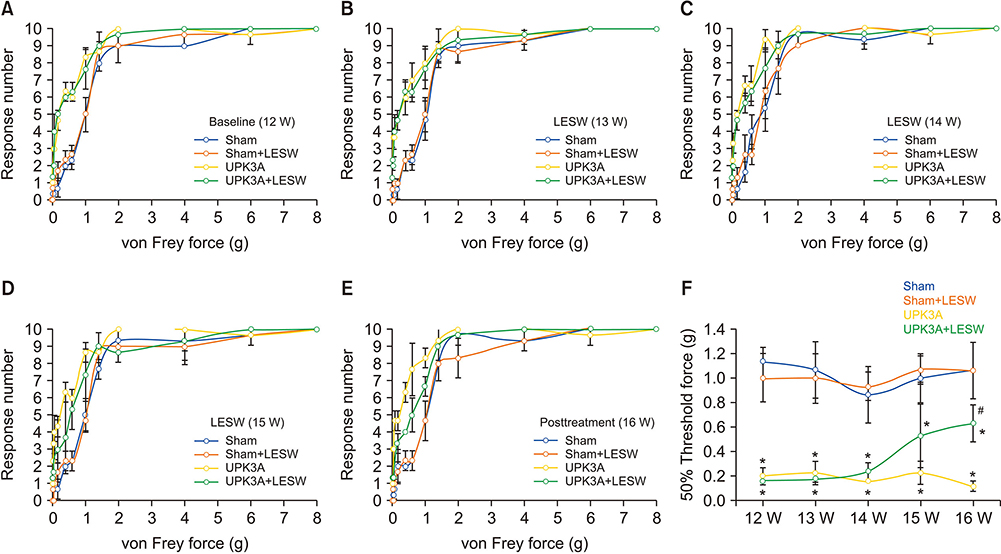
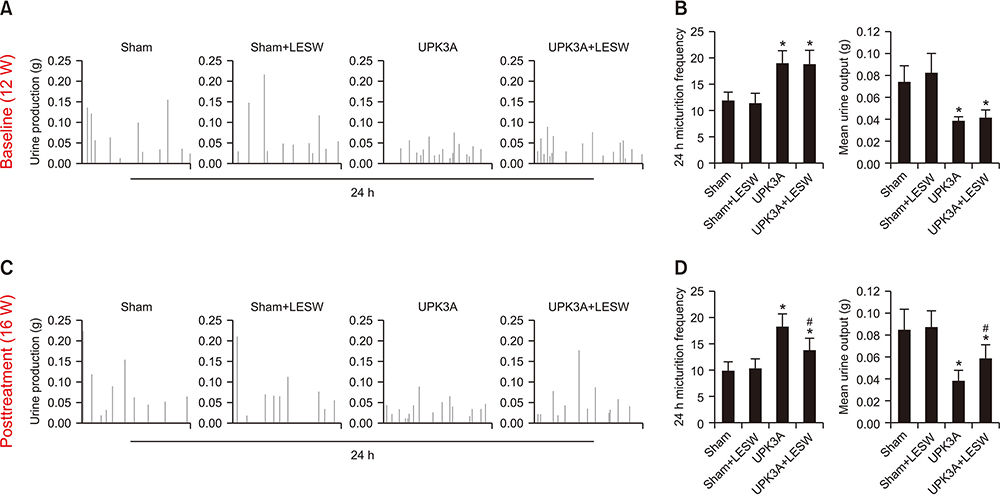
Forty female BALB/c mice were divided into four groups (n=10/group): Sham, Sham+LESW, UPK3A, and UPK3A+LESW. At 6 weeks of age, mice were injected with an emulsion containing water and complete Freund's adjuvant with (UPK3A and UPK3A+LESW groups) or without (Sham and Sham+LESW groups) 200 µg of UPK3A. At 10 weeks, mice received a second dose of Freund's adjuvant to booster immunization. At 12 weeks, mice underwent pain assessment and a frequency volume chart (FVC) test as the pretreatment assessment. LESW treatment and pain assessment were conducted from 13 to 15 weeks. One week after the final treatment, pain assessment and the FVC were conducted again as the post-treatment assessment. Mice were euthanized and sacrificed at 17 weeks.
RESULTS
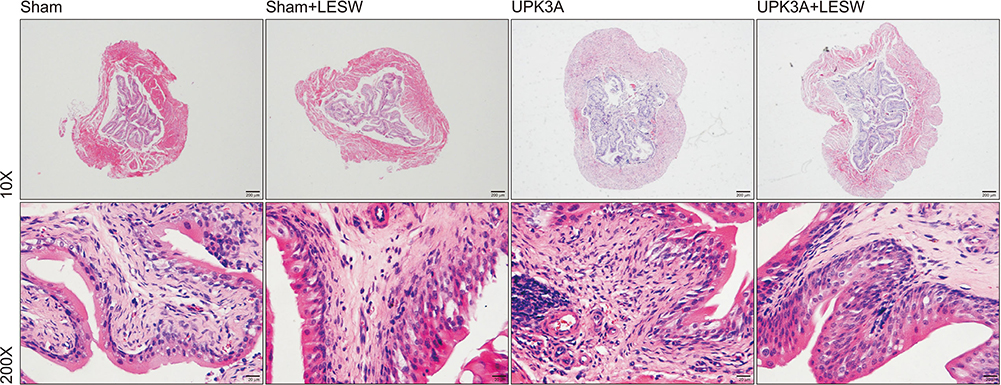
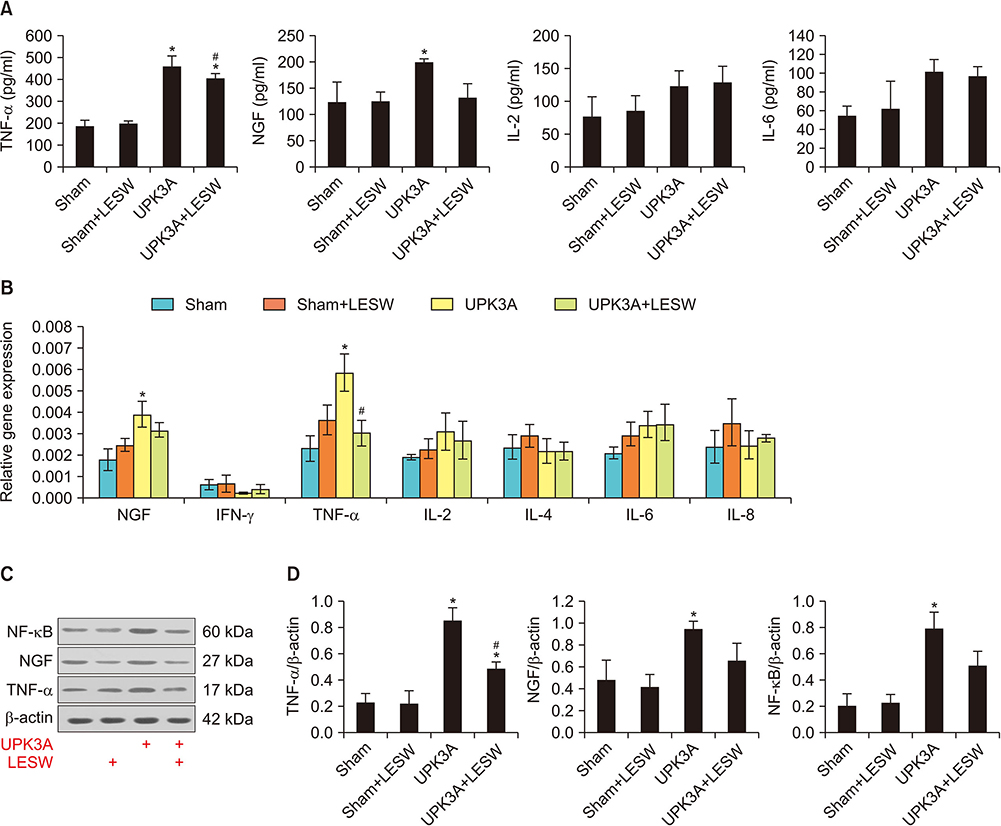
The presence of tactile allodynia and bladder dysfunction was significant in the UPK3A-injected mice. LESW raised the pain threshold and improved bladder function with decreased urinary frequency and increased mean urine output. Expression and secretion of local and systemic inflammatory markers, including tumor necrosis factor-α (TNF-α) and nerve growth factor (NGF), increased after UPK3A immunization. These markers were significantly decreased after LESW treatment (p<0.05).
CONCLUSIONS
LESW treatment attenuated pain and bladder dysfunction in a UPK3A-induced model of IC/PBS. Local and systemic inflammation was partially controlled, with a reduced number of infiltrated inflammatory cells and reduced levels of TNF-α and NGF.
MeSH Terms
-
Animals*
Cystitis, Interstitial
Female
Freund's Adjuvant
High-Energy Shock Waves
Humans
Hyperalgesia
Immunization
Immunization, Secondary
Inflammation
Mice
Models, Animal*
Necrosis
Nerve Growth Factor
Pain Measurement
Pain Threshold
Shock*
Urinary Bladder*
Uroplakins*
Water
Freund's Adjuvant
Nerve Growth Factor
Uroplakins
Water
Figure
Reference
-
1. Bicer F, Altuntas CZ, Izgi K, Ozer A, Kavran M, Tuohy VK, et al. Chronic pelvic allodynia is mediated by CCL2 through mast cells in an experimental autoimmune cystitis model. Am J Physiol Renal Physiol. 2015; 308:F103–F113.
Article2. Tonyali S, Ates D, Akbiyik F, Kankaya D, Baydar D, Ergen A. Urine nerve growth factor (NGF) level, bladder nerve staining and symptom/problem scores in patients with interstitial cystitis. Adv Clin Exp Med. 2018; 27:159–163.
Article3. Berry SH, Elliott MN, Suttorp M, Bogart LM, Stoto MA, Eggers P, et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol. 2011; 186:540–544.
Article4. Link CL, Pulliam SJ, Hanno PM, Hall SA, Eggers PW, Kusek JW, et al. Prevalence and psychosocial correlates of symptoms suggestive of painful bladder syndrome: results from the Boston area community health survey. J Urol. 2008; 180:599–606.
Article5. Altuntas CZ, Daneshgari F, Sakalar C, Goksoy E, Gulen MF, Kavran M, et al. Autoimmunity to uroplakin II causes cystitis in mice: a novel model of interstitial cystitis. Eur Urol. 2012; 61:193–200.
Article6. Nickel JC, Egerdie B, Davis E, Evans R, Mackenzie L, Shrewsbury SB. A phase II study of the efficacy and safety of the novel oral SHIP1 activator AQX-1125 in subjects with moderate to severe interstitial cystitis/bladder pain syndrome. J Urol. 2016; 196:747–754.
Article7. Dellis AE, Papatsoris AG. Bridging pharmacotherapy and minimally invasive surgery in interstitial cystitis/bladder pain syndrome treatment. Expert Opin Pharmacother. 2018; 19:1369–1373.
Article8. Kong XT, Deng FM, Hu P, Liang FX, Zhou G, Auerbach AB, et al. Roles of uroplakins in plaque formation, umbrella cell enlargement, and urinary tract diseases. J Cell Biol. 2004; 167:1195–1204.
Article9. Li H, Matheu MP, Sun F, Wang L, Sanford MT, Ning H, et al. Low-energy shock wave therapy ameliorates erectile dysfunction in a pelvic neurovascular injuries rat model. J Sex Med. 2016; 13:22–32.
Article10. Abe Y, Ito K, Hao K, Shindo T, Ogata T, Kagaya Y, et al. Extracorporeal low-energy shock-wave therapy exerts anti-inflammatory effects in a rat model of acute myocardial infarction. Circ J. 2014; 78:2915–2925.
Article11. Al-Abbad H, Simon JV. The effectiveness of extracorporeal shock wave therapy on chronic achilles tendinopathy: a systematic review. Foot Ankle Int. 2013; 34:33–41.12. Angehrn F, Kuhn C, Voss A. Can cellulite be treated with low-energy extracorporeal shock wave therapy? Clin Interv Aging. 2007; 2:623–630.
Article13. Paoloni M, Tavernese E, Cacchio A, D'orazi V, Ioppolo F, Fini M, et al. Extracorporeal shock wave therapy and ultrasound therapy improve pain and function in patients with carpal tunnel syndrome. A randomized controlled trial. Eur J Phys Rehabil Med. 2015; 51:521–528.14. Done JD, Rudick CN, Quick ML, Schaeffer AJ, Thumbikat P. Role of mast cells in male chronic pelvic pain. J Urol. 2012; 187:1473–1482.
Article15. Theoharides TC, Kempuraj D, Sant GR. Mast cell involvement in interstitial cystitis: a review of human and experimental evidence. Urology. 2001; 57(6 Suppl 1):47–55.
Article16. Seth P, Scholl L, Rudd RA, Bacon S. Overdose deaths involving opioids, cocaine, and psychostimulants-United States, 2015–2016. MMWR Morb Mortal Wkly Rep. 2018; 67:349–358.17. Gao F, Chen N, Sun W, Wang B, Shi Z, Cheng L, et al. Combined therapy with shock wave and retrograde bone marrow-derived cell transplantation for osteochondral lesions of the talus. Sci Rep. 2017; 7:2106.
Article18. Yang W, He Y, Gan L, Zhang F, Hua B, Yang P, et al. Cardiac shock wave therapy promotes arteriogenesis of coronary micrangium, and ILK is involved in the biomechanical effects by proteomic analysis. Sci Rep. 2018; 8:1814.
Article19. Xu JK, Chen HJ, Li XD, Huang ZL, Xu H, Yang HL, et al. Optimal intensity shock wave promotes the adhesion and migration of rat osteoblasts via integrin β1-mediated expression of phosphorylated focal adhesion kinase. J Biol Chem. 2012; 287:26200–26212.
Article20. Liu HT, Tyagi P, Chancellor MB, Kuo HC. Urinary nerve growth factor level is increased in patients with interstitial cystitis/bladder pain syndrome and decreased in responders to treatment. BJU Int. 2009; 104:1476–1481.
Article21. Nayak R, Banik RK. Current innovations in peripheral nerve stimulation. Pain Res Treat. 2018; 2018:9091216.
Article22. Hand JR. Interstitial cystitis; report of 223 cases (204 women and 19 men). J Urol. 1949; 61:291–310.
Article23. Christmas TJ, Rode J, Chapple CR, Milroy EJ, Turner-Warwick RT. Nerve fibre proliferation in interstitial cystitis. Virchows Arch A Pathol Anat Histopathol. 1990; 416:447–451.
Article24. Pang X, Marchand J, Sant GR, Kream RM, Theoharides TC. Increased number of substance P positive nerve fibres in interstitial cystitis. Br J Urol. 1995; 75:744–750.
Article25. Hohenfellner M, Nunes L, Schmidt RA, Lampel A, Thüroff JW, Tanagho EA. Interstitial cystitis: increased sympathetic innervation and related neuropeptide synthesis. J Urol. 1992; 147:587–591.
Article26. Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965; 150:971–979.
Article27. Ulloa L, Quiroz-Gonzalez S, Torres-Rosas R. Nerve stimulation: immunomodulation and control of inflammation. Trends Mol Med. 2017; 23:1103–1120.
Article28. National Research Council (US) Committee on Recognition and Alleviation of Pain in Laboratory Animals. Recognition and alleviation of pain in laboratory animals. National Academy of Sciences (US). The National Academies collection: reports funded by National Institutes of Health. Washington, DC: National Academies Press;2009.29. Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007; 55:377–391.
Article30. Almeida TCDC, Figueiredo FWDS, Barbosa Filho VC, de Abreu LC, Fonseca FLA, Adami F. Effects of transcutaneous electrical nerve stimulation (TENS) on proinflammatory cytokines: protocol for systematic review. Syst Rev. 2017; 6:139.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Commentary on “New Frontiers or the Treatment of Interstitial Cystitis/Bladder Pain Syndrome-Focused on Stem Cells, Platelet-Rich Plasma, and Low-Energy Shock Wave”
- Diagnosis and treatment of interstitial cystitis and painful bladder syndrome
- The Effect of Lumbar Sympathetic Block in Interstitial Cystitis: A case report
- New Frontiers or the Treatment of Interstitial Cystitis/Bladder Pain Syndrome - Focused on Stem Cells, Platelet-Rich Plasma, and Low-Energy Shock Wave
- Interstitial Cystitis/Painful Bladder Syndrome: Prevalence Estimates, Quality of Life and Depression among Older Adult Korean Women