Korean J Physiol Pharmacol.
2019 Sep;23(5):419-426. 10.4196/kjpp.2019.23.5.419.
Gastroprokinetic agent, mosapride inhibits 5-HT₃ receptor currents in NCB-20 cells
- Affiliations
-
- 1Department of Anatomy, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea.
- 2Department of Pharmacology, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea. sungkw@catholic.ac.kr
- KMID: 2455818
- DOI: http://doi.org/10.4196/kjpp.2019.23.5.419
Abstract
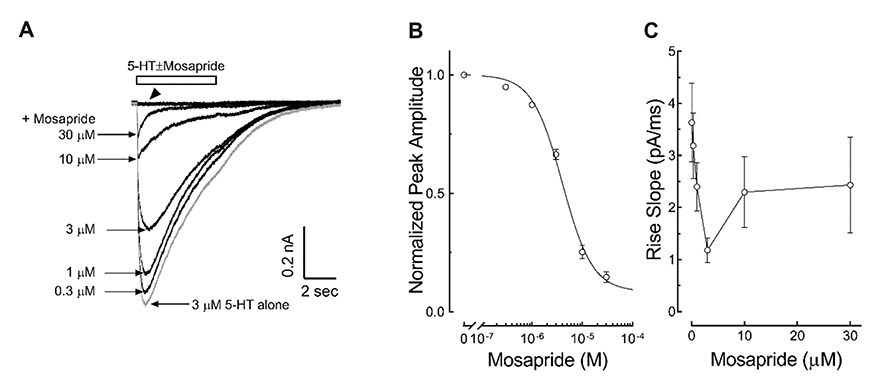
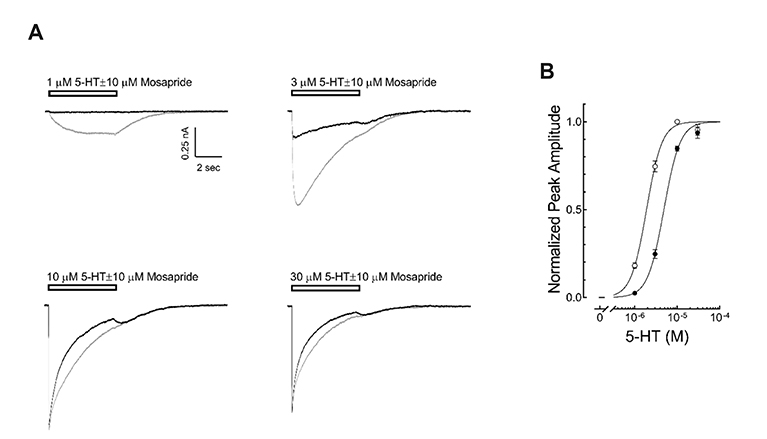
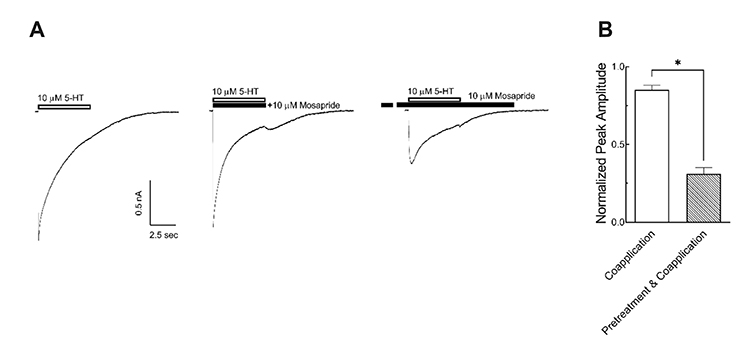
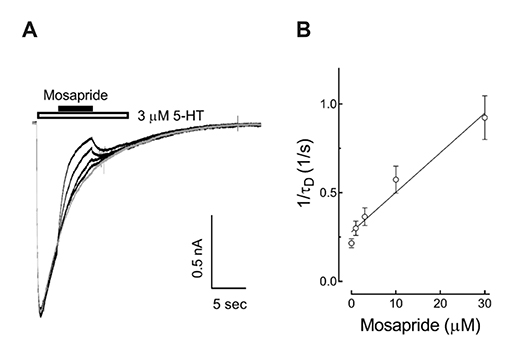
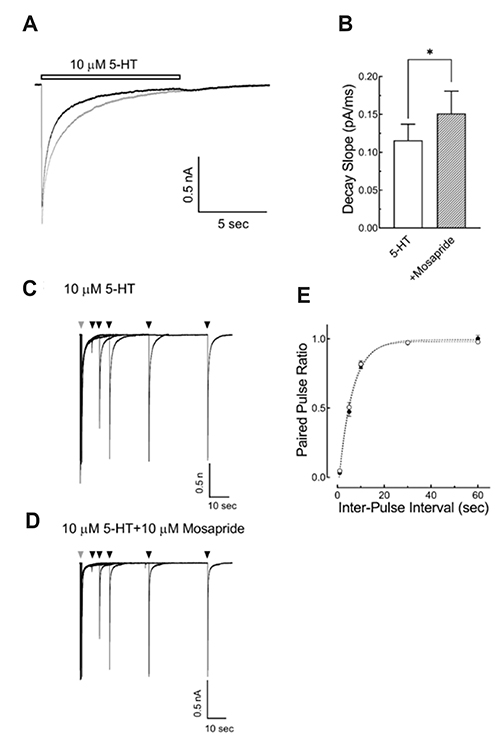
- Mosapride accelerates gastric emptying by acting on 5-hydroxytryptamine type 4 (5-HT₄) receptor and is frequently used in the treatment of gastrointestinal (GI) disorders requiring gastroprokinetic efficacy. We tested the effect of mosapride on 5-hydroxytryptamine type 3 (5-HT₃) receptor currents because the 5-HT₃ receptors are also known to be expressed in the GI system and have an important role in the regulation of GI functions. Using the whole-cell voltage clamp method, we compared the currents of the 5-HT₃ receptors when 5-HT was applied alone or was co-applied with mosapride in cultured NCB-20 cells known to express 5-HT₃ receptors. The 5-HT₃ receptor current amplitudes were inhibited by mosapride in a concentration-dependent manner. Mosapride blocked the peak currents evoked by the application of 5-HT in a competitive manner because the EC₅₀ shifted to the right without changing the maximal effect. The rise slopes of 5-HT₃ receptor currents were decreased by mosapride. Pre-application of mosapride before co-application, augmented the inhibitory effect of mosapride, which suggests a closed channel blocking mechanism. Mosapride also blocked the opened 5-HT₃ receptor because it inhibited the 5-HT₃ receptor current in the middle of the application of 5-HT. It accelerated desensitization of the 5-HT₃ receptor but did not change the recovery process from the receptor desensitization. There were no voltage-, or use-dependency in its blocking effects. These results suggest that mosapride inhibited the 5-HT₃ receptor through a competitive blocking mechanism probably by binding to the receptor in closed state, which could be involved in the pharmacological effects of mosapride to treat GI disorders.
MeSH Terms
Figure
Reference
-
1. Yoshida N, Ito T, Karasawa T, Itoh Z. AS-4370, a new gastrokinetic agent, enhances upper gastrointestinal motor activity in conscious dogs. J Pharmacol Exp Ther. 1991; 257:781–787.2. Curran MP, Robinson DM. Mosapride in gastrointestinal disorders. Drugs. 2008; 68:981–991.3. Yoshikawa T, Yoshida N, Mine Y, Hosoki K. Affinity of mosapride citrate, a new gastroprokinetic agent, for 5-HT4 receptors in guinea pig ileum. Jpn J Pharmacol. 1998; 77:53–59.4. Wade PR, Mawe GM, Branchek TA, Gershon MD. Use of stereoisomers of zacopride to analyze actions of 5-hydroxytryptamine on enteric neurons. Am J Physiol. 1991; 260(1 Pt 1):G80–G90.
Article5. Nemeth PR, Ort CA, Zafirov DH, Wood JD. Interactions between serotonin and cisapride on myenteric neurons. Eur J Pharmacol. 1985; 108:77–83.
Article6. Nemeth PR, Gullikson GW. Gastrointestinal motility stimulating drugs and 5-HT receptors on myenteric neurons. Eur J Pharmacol. 1989; 166:387–391.
Article7. Wu ZS, Cheng H, Jiang Y, Melcher K, Xu HE. Ion channels gated by acetylcholine and serotonin: structures, biology, and drug discovery. Acta Pharmacol Sin. 2015; 36:895–907.
Article8. Sugita S, Shen KZ, North RA. 5-hydroxytryptamine is a fast excitatory transmitter at 5-HT3 receptors in rat amygdala. Neuron. 1992; 8:199–203.9. Morales M, Battenberg E, de Lecea L, Sanna PP, Bloom FE. Cellular and subcellular immunolocalization of the type 3 serotonin receptor in the rat central nervous system. Brain Res Mol Brain Res. 1996; 36:251–260.
Article10. Holbrook JD, Gill CH, Zebda N, Spencer JP, Leyland R, Rance KH, Trinh H, Balmer G, Kelly FM, Yusaf SP, Courtenay N, Luck J, Rhodes A, Modha S, Moore SE, Sanger GJ, Gunthorpe MJ. Characterisation of 5-HT3C, 5-HT3D and 5-HT3E receptor subunits: evolution, distribution and function. J Neurochem. 2009; 108:384–396.11. Miyake A, Mochizuki S, Takemoto Y, Akuzawa S. Molecular cloning of human 5-hydroxytryptamine3 receptor: heterogeneity in distribution and function among species. Mol Pharmacol. 1995; 48:407–416.12. Akuzawa S, Miyake A, Miyata K, Fukutomi H. Comparison of [3H] YM060 binding to native and cloned rat 5-HT3 receptors. Eur J Pharmacol. 1996; 296:227–230.13. Walstab J, Rappold G, Niesler B. 5-HT3 receptors: role in disease and target of drugs. Pharmacol Ther. 2010; 128:146–169.14. Thompson AJ. Recent developments in 5-HT3 receptor pharmacology. Trends Pharmacol Sci. 2013; 34:100–109.15. Fakhfouri G, Rahimian R, Ghia JE, Khan WI, Dehpour AR. Impact of 5-HT3 receptor antagonists on peripheral and central diseases. Drug Discov Today. 2012; 17:741–747.16. Machu TK. Therapeutics of 5-HT3 receptor antagonists: current uses and future directions. Pharmacol Ther. 2011; 130:338–347.17. Zheng Y, Yu T, Tang Y, Xiong W, Shen X, Jiang L, Lin L. Efficacy and safety of 5-hydroxytryptamine 3 receptor antagonists in irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2017; 12:e0172846.
Article18. Park YS, Myeong SH, Kim IB, Sung KW. Tricyclic antidepressant amitriptyline inhibits 5-hydroxytryptamine 3 receptor currents in NCB-20 cells. Korean J Physiol Pharmacol. 2018; 22:585–595.
Article19. Snyders D, Hondeghem L, Bennett P. Mechanisms of drug-channel interaction. In : Fozzard HA, Haber E, Jennings RB, Katz AM, Morgan HE, editors. The heart and cardiovascular system: scientific foundations, vol. 2. New York: Raven Press;1992.20. Zhou Q, Verdoorn TA, Lovinger DM. Alcohols potentiate the function of 5-HT3 receptor-channels on NCB-20 neuroblastoma cells by favouring and stabilizing the open channel state. J Physiol. 1998; 507 (Pt 2):335–352.21. van Hooft JA, van der Haar E, Vijverberg HP. Allosteric potentiation of the 5-HT3 receptor-mediated ion current in N1E-115 neuroblastoma cells by 5-hydroxyindole and analogues. Neuropharmacology. 1997; 36:649–653.22. Neijt HC, Plomp JJ, Vijverberg HP. Kinetics of the membrane current mediated by serotonin 5-HT3 receptors in cultured mouse neuroblastoma cells. J Physiol. 1989; 411:257–269.23. Gunthorpe MJ, Lummis SC. Diltiazem causes open channel block of recombinant 5-HT3 receptors. J Physiol. 1999; 519 Pt 3:713–722.24. Nayak SV, Rondé P, Spier AD, Lummis SC, Nichols RA. Calcium changes induced by presynaptic 5-hydroxytryptamine-3 serotonin receptors on isolated terminals from various regions of the rat brain. Neuroscience. 1999; 91:107–117.
Article25. Thompson AJ, Lummis SC. 5-HT3 receptors. Curr Pharm Des. 2006; 12:3615–3630.26. Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007; 132:397–414.
Article27. Gregory RE, Ettinger DS. 5-HT3 receptor antagonists for the prevention of chemotherapy-induced nausea and vomiting A comparison of their pharmacology and clinical efficacy. Drugs. 1998; 55:173–189.28. Galligan JJ, LePard KJ, Schneider DA, Zhou X. Multiple mechanisms of fast excitatory synaptic transmission in the enteric nervous system. J Auton Nerv Syst. 2000; 81:97–103.
Article29. Mawe GM, Hoffman JM. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013; 10:473–486.
Article30. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006; 130:1480–1491.
Article31. Lazaraki G, Chatzimavroudis G, Katsinelos P. Recent advances in pharmacological treatment of irritable bowel syndrome. World J Gastroenterol. 2014; 20:8867–8885.32. Karasawa T, Yoshida N, Furukawa K, Omoya H, Ito T. Comparison of gastrokinetic effects of AS-4370, cisapride and BRL24924. Eur J Pharmacol. 1990; 183:2181.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lamotrigine, an antiepileptic drug, inhibits 5-HT₃ receptor currents in NCB-20 neuroblastoma cells
- Selective serotonin reuptake inhibitor escitalopram inhibits 5-HT₃ receptor currents in NCB-20 cells
- Tricyclic antidepressant amitriptyline inhibits 5-hydroxytryptamine 3 receptor currents in NCB-20 cells
- Investigation into the Effects of Mosapride on Motility of Guinea Pig Stomach, Ileum, and Colon
- Eugenol Inhibits ATP-induced P2X Currents in Trigeminal Ganglion Neurons