Efficacy and safety of abrilumab, an α4β7 integrin inhibitor, in Japanese patients with moderate-to-severe ulcerative colitis: a phase II study
- Affiliations
-
- 1Center for Advanced IBD Research and Treatment, Kitasato University Kitasato Institute Hospital, Kitasato University, Tokyo, Japan. thibi@insti.kitasato-u.ac.jp
- 2IBD Center, Sapporo-Kosei General Hospital, Sapporo, Japan.
- 3IBD Center, Sapporo Tokushukai Hospital, Sapporo, Japan.
- 4Department of Gastroenterology and Proctology, Sai Gastroenterology/Proctology Clinic, Osaka, Japan.
- 5Department of Internal Medicine, Sameshima Hospital, Kagoshima, Japan.
- 6Department of Inflammatory Bowel Disease, Division of Internal Medicine, Hyogo College of Medicine Hospital, Nishinomiya, Japan.
- 7IBD Center, Sapporo Higashi Tokushukai Hospital, Sapporo, Japan.
- 8Research & Development, AstraZeneca K.K., Osaka, Japan.
- 9Amgen, South San Francisco, CA, USA.
- 10MedImmune LLC, Gaithersburg, MD, USA.
- 11Department of Internal Medicine, Toho University Sakura Medical Center, Sakura, Japan.
- KMID: 2454746
- DOI: http://doi.org/10.5217/ir.2018.00141
Abstract
- BACKGROUND/AIMS
Inhibition of α4β7 integrin has been shown to be effective for induction and maintenance therapy in patients with ulcerative colitis (UC). We investigated the effects of varying doses of the α4β7 inhibitor abrilumab in Japanese patients with moderate-to-severe UC despite conventional treatments.
METHODS
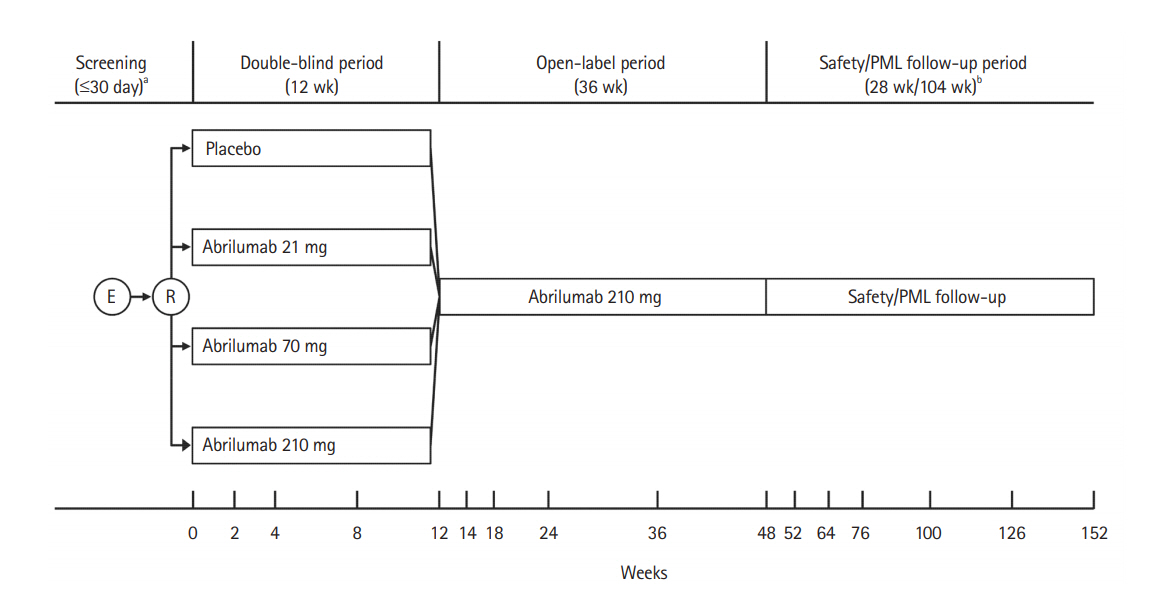
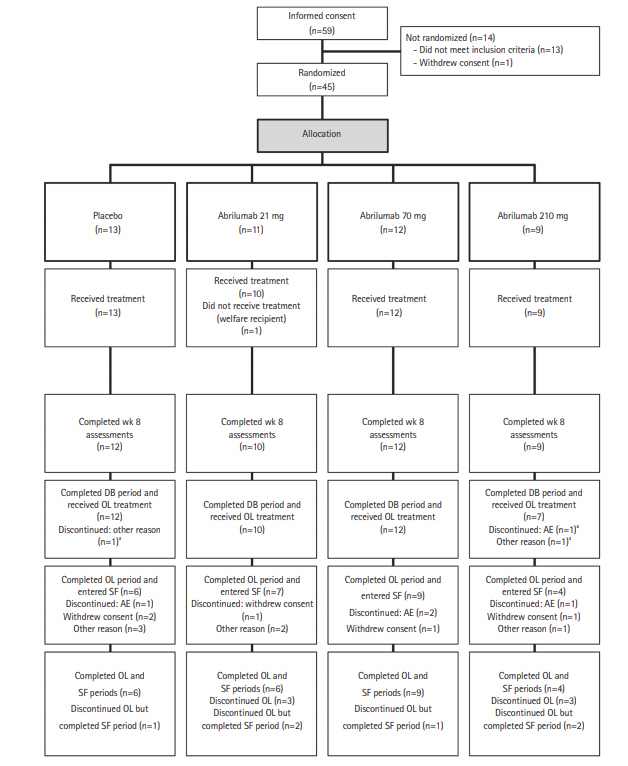
In this randomized, double-blind, placebo-controlled study, 45 UC patients were randomized to abrilumab 21 mg (n=11), 70 mg (n=12), 210 mg (n=9), or placebo (n=13) via subcutaneous (SC) injection for 12 weeks. The double-blind period was followed by a 36-week open-label period, in which all patients received abrilumab 210 mg SC every 12 weeks, and a 28-week safety follow-up period. The primary efficacy variable was clinical remission at week 8 (total Mayo score ≤2 points with no individual subscore >1 point).
RESULTS
Clinical remission at week 8 was 4 out of 31 (12.9%) overall in the abrilumab groups versus 0 out of 13 in the placebo group (abrilumab 21 mg, 1/10 [10.0%]; 70 mg, 2/12 [16.7%]; 210 mg, 1/9 [11.1%]). In both the double-blind and open-label periods, fewer patients in the abrilumab groups experienced ≥1 adverse event compared with those in the placebo group. There were no cases of progressive multifocal leukoencephalopathy and no deaths.
CONCLUSIONS
Abrilumab 70 mg and 210 mg yielded numerically better results in terms of clinical remission rate at Week 8 than placebo, with the 210 mg dose showing more consistent treatment effects. Abrilumab was well tolerated in Japanese patients with UC.
Keyword
MeSH Terms
Figure
Cited by 3 articles
-
Is fasting beneficial for hospitalized patients with inflammatory bowel diseases?
Yong Eun Park, Yehyun Park, Soo Jung Park, Tae Il Kim, Won Ho Kim, Jung Nam Kim, Na Rae Lee, Jae Hee Cheon
Intest Res. 2020;18(1):85-95. doi: 10.5217/ir.2019.00055.Efficacy and safety of a new vedolizumab subcutaneous formulation in Japanese patients with moderately to severely active ulcerative colitis
Taku Kobayashi, Hiroaki Ito, Toshifumi Ashida, Tadashi Yokoyama, Masakazu Nagahori, Tomoki Inaba, Mitsuhiro Shikamura, Takayoshi Yamaguchi, Tetsuharu Hori, Philippe Pinton, Mamoru Watanabe, Toshifumi Hibi
Intest Res. 2021;19(4):448-460. doi: 10.5217/ir.2020.00026.Subcutaneous integrin inhibitors may provide more treatment options for patients with moderate-to-severe ulcerative colitis
Hyuk Yoon
Intest Res. 2019;17(3):283-284. doi: 10.5217/ir.2019.00070.
Reference
-
1. Ministry of Health, Labour and Welfare: Shared Study Report FY 2016 on “Research on intractable inflammatory bowel disease” (Suzuki Group). Ulcerative colitis/Crohn’s disease diagnostic criteria/treatment guideline. http://www.ibdjapan.org/pdf/doc01.pdf. Accessed May 5, 2018.2. Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011; 140:1785–1794.
Article3. Prideaux L, Kamm MA, De Cruz PP, Chan FK, Ng SC. Inflammatory bowel disease in Asia: a systematic review. J Gastroenterol Hepatol. 2012; 27:1266–1280.
Article4. 2010 Public Health Report (Table 65 No. of patients receiving medical treatment for refractory diseases by sex, age group and disease). Ministry of Health, Labour and Welfare Web site. http://www.mhlw.go.jp/english/index.html. Accessed May 6, 2018.5. Asakura K, Nishiwaki Y, Inoue N, Hibi T, Watanabe M, Takebayashi T. Prevalence of ulcerative colitis and Crohn’s disease in Japan. J Gastroenterol. 2009; 44:659–665.
Article6. Hoie O, Wolters F, Riis L, et al. Ulcerative colitis: patient characteristics may predict 10-yr disease recurrence in a European-wide population-based cohort. Am J Gastroenterol. 2007; 102:1692–1701.
Article7. Hiwatashi N, Yao T, Watanabe H, et al. Long-term follow-up study of ulcerative colitis in Japan. J Gastroenterol. 1995; 30 Suppl 8:13–16.8. Kornbluth A, Sachar DB; Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010; 105:501–523.
Article9. Lichtenstein GR, Abreu MT, Cohen R, Tremaine W; American Gastroenterological Association. American Gastroenterological Association Institute medical position statement on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology. 2006; 130:935–939.
Article10. Suzuki Y, Motoya S, Hanai H, et al. Efficacy and safety of adalimumab in Japanese patients with moderately to severely active ulcerative colitis. J Gastroenterol. 2014; 49:283–294.
Article11. Matsuoka K, Kobayashi T, Ueno F, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol. 2018; 53:305–353.
Article12. Arihiro S, Ohtani H, Suzuki M, et al. Differential expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in ulcerative colitis and Crohn’s disease. Pathol Int. 2002; 52:367–374.
Article13. Briskin M, Winsor-Hines D, Shyjan A, et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol. 1997; 151:97–110.14. Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013; 369:699–710.
Article15. Hesterberg PE, Winsor-Hines D, Briskin MJ, et al. Rapid resolution of chronic colitis in the cotton-top tamarin with an antibody to a gut-homing integrin alpha 4 beta 7. Gastroenterology. 1996; 111:1373–1380.
Article16. Picarella D, Hurlbut P, Rottman J, Shi X, Butcher E, Ringler DJ. Monoclonal antibodies specific for beta 7 integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) reduce inflammation in the colon of scid mice reconstituted with CD45RBhigh CD4+ T cells. J Immunol. 1997; 158:2099–2106.
Article17. Sandborn WJ, Cyrille M, Hansen MB, et al. Efficacy and safety of abrilumab in a randomized, placebo-controlled trial for moderate to severe ulcerative colitis. Gastroenterology. [published online ahead of print November 22, 2018]. https://doi.org/10.1053/j.gastro.2018.11.035.
Article18. Guyatt G, Mitchell A, Irvine EJ, et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology. 1989; 96:804–810.
Article19. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis: a randomized study. N Engl J Med. 1987; 317:1625–1629.
Article20. Feagan BG, Greenberg GR, Wild G, et al. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N Engl J Med. 2005; 352:2499–2507.
Article21. Yang SK, Hong M, Baek J, et al. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet. 2014; 46:1017–1020.
Article22. Zhu X, Wang XD, Chao K, et al. NUDT15 polymorphisms are better than thiopurine S-methyltransferase as predictor of risk for thiopurine-induced leukopenia in Chinese patients with Crohn’s disease. Aliment Pharmacol Ther. 2016; 44:967–975.
Article23. Yang JJ, Landier W, Yang W, et al. Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J Clin Oncol. 2015; 33:1235–1242.
Article24. Kobayashi T, Hisamatsu T, Suzuki Y, et al. Predicting outcomes to optimize disease management in inflammatory bowel disease in Japan: their differences and similarities to Western countries. Intest Res. 2018; 16:168–177.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Subcutaneous integrin inhibitors may provide more treatment options for patients with moderate-to-severe ulcerative colitis
- Old and New Biologics and Small Molecules in Inflammatory Bowel Disease: Anti Integrins
- Advancements in the Management of Moderate-to-Severe Ulcerative Colitis: A Revised 2023 Korean Treatment Guidelines
- Golimumab Therapy in Ulcerative Colitis
- The Efficacy of Vedolizumab, an alpha4beta7 Integrin Antibody, in Crohn's Disease and Ulcerative Colitis