Ann Clin Neurophysiol.
2018 Jul;20(2):57-65. 10.14253/acn.2018.20.2.57.
Application of near-infrared spectroscopy in clinical neurology
- Affiliations
-
- 1Department of Neurology, Hangang Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea.
- 2Department of Neurology, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea.
- 3Department of Neurology, Kangdong Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea. jsb_res@hotmail.co.kr
- KMID: 2454706
- DOI: http://doi.org/10.14253/acn.2018.20.2.57
Abstract
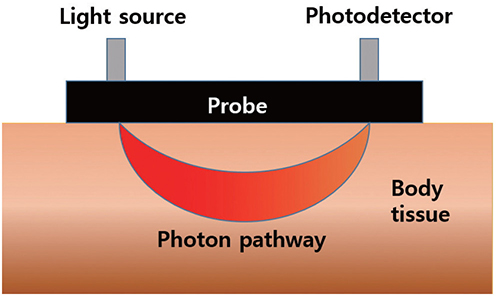
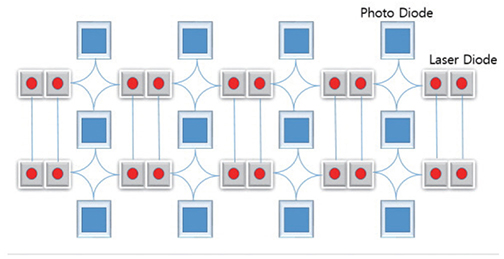
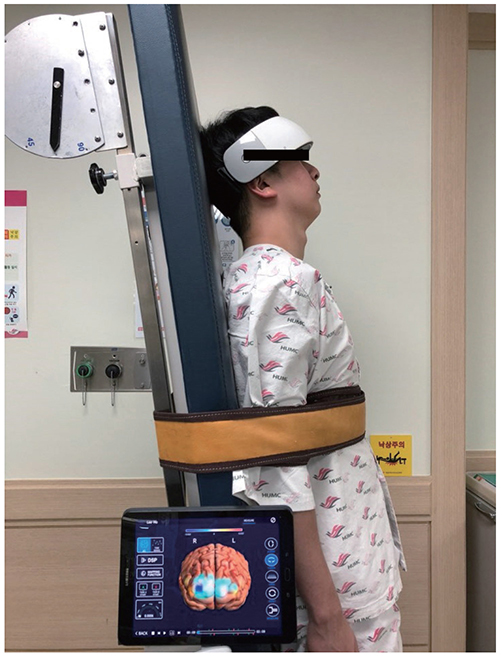
- Near-infrared spectroscopy (NIRS) monitoring has been used mainly to detect reduced perfusion of the brain during orthostatic stress in order to assess orthostatic intolerance (OI). Many studies have investigated the use of NIRS to reveal the pathophysiology of patients with OI. Research using NIRS in other neurological diseases (e.g., stroke, epilepsy, and migraine) is continuing. NIRS may play an important role in monitoring the regional distribution of the hemodynamic flow in real time and thereby reveal the underlying pathophysiology and facilitate the management of not only patients with OI symptoms but also those with various neurological diseases.
MeSH Terms
Figure
Reference
-
1. Misgeld T, Kerschensteiner M. In vivo imaging of the diseased nervous system. Nat Rev Neurosci. 2006; 7:449–463.
Article2. Kim HY, Seo K, Jeon HJ, Lee U, Lee H. Application of functional near-infrared spectroscopy to the study of brain function in humans and animal models. Mol Cells. 2017; 40:523–532.
Article3. Irani F, Platek SM, Bunce S, Ruocco AC, Chute D. Functional near infrared spectroscopy (fNIRS): an emerging neuroimaging technology with important applications for the study of brain disorders. Clin Neuropsychol. 2007; 21:9–37.
Article4. Izzetoglu M, Izzetoglu K, Bunce S, Ayaz H, Devaraj A, Onaral B, et al. Functional near-infrared neuroimaging. IEEE Trans Neural Syst Rehabil Eng. 2005; 13:153–159.
Article5. Strangman G, Culver JP, Thompson JH, Boas DA. A quantitative comparison of simultaneous BOLD fMRI and NIRS recordings during functional brain activation. Neuroimage. 2002; 17:719–731.
Article6. Jöbsis FF. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 1977; 198:1264–1267.
Article7. Villringer A, Chance B. Non-invasive optical spectroscopy and imaging of human brain function. Trends Neurosci. 1997; 20:435–442.
Article8. Strangman G, Boas DA, Sutton JP. Non-invasive neuroimaging using near-infrared light. Biol Psychiatry. 2002; 52:679–693.
Article9. Bunce SC, Izzetoglu M, Izzetoglu K, Onaral B, Pourrezaei K. Functional near-infrared spectroscopy. IEEE Eng Med Biol Mag. 2006; 25:54–62.
Article10. Gratton G, Brumback CR, Gordon BA, Pearson MA, Low KA, Fabiani M. Effects of measurement method, wavelength, and source-detector distance on the fast optical signal. Neuroimage. 2006; 32:1576–1590.
Article11. Ferrari M, Quaresima V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. Neuroimage. 2012; 63:921–935.
Article12. Okada E, Firbank M, Schweiger M, Arridge SR, Cope M, Delpy DT. Theoretical and experimental investigation of near-infrared light propagation in a model of the adult head. Appl Opt. 1997; 36:21–31.
Article13. Strangman G, Franceschini MA, Boas DA. Factors affecting the accuracy of near-infrared spectroscopy concentration calculations for focal changes in oxygenation parameters. Neuroimage. 2003; 18:865–879.
Article14. Sakatani K, Chen S, Lichty W, Zuo H, Wang YP. Cerebral blood oxygenation changes induced by auditory stimulation in newborn infants measured by near infrared spectroscopy. Early Hum Dev. 1999; 55:229–236.
Article15. Liu H, Song Y, Worden KL, Jiang X, Constantinescu A, Mason RP. Noninvasive investigation of blood oxygenation dynamics of tumors by near-infrared spectroscopy. Appl Opt. 2000; 39:5231–5243.
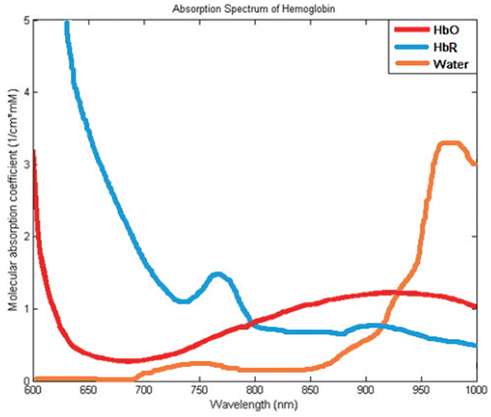
Article16. Optical absorption of hemoglobin [Internet]. Portland (OR, USA): Scott Prahl, Oregon Medical Laser Center;c2018. accessed 2018 Jun 26. Available from: https://omlc.org/spectra/hemoglobin/.17. Rothschild AH, Weinberg CR, Halter JB, Porte D Jr, Pfeifer MA. Sensitivity of R-R variation and valsalva ratio in assessment of cardiovascular diabetic autonomic neuropathy. Diabetes Care. 1987; 10:735–741.
Article18. Goto T, Kita Y, Suzuki K, Koike T, Inagaki M. Lateralized frontal activity for Japanese phonological processing during child development. Front Hum Neurosci. 2015; 9:417.
Article19. Kita Y, Gunji A, Inoue Y, Goto T, Sakihara K, Kaga M, et al. Self-face recognition in children with autism spectrum disorders: a near-infrared spectroscopy study. Brain Dev. 2011; 33:494–503.
Article20. Shen WK, Sheldon RS, Benditt DG, Cohen MI, Forman DE, Goldberger ZD, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2017; 14:e155–e217.21. Benarroch EE. The clinical approach to autonomic failure in neurological disorders. Nat Rev Neurol. 2014; 10:396–407.
Article22. Shields RW Jr. Autonomic nervous system testing. In : Levin K, Luders HO, editors. Comprehensive clinical neurophysiology. 1st ed. Philadelphia: Saunders;2000. p. 307–324.23. Wang B, Zhang M, Bu L, Xu L, Wang W, Li Z. Posture-related changes in brain functional connectivity as assessed by wavelet phase coherence of NIRS signals in elderly subjects. Behav Brain Res. 2016; 312:238–245.
Article24. Yücel MA, Selb J, Aasted CM, Petkov MP, Becerra L, Borsook D, et al. Short separation regression improves statistical significance and better localizes the hemodynamic response obtained by near-infrared spectroscopy for tasks with differing autonomic responses. Neurophotonics. 2015; 2:035005.
Article25. Stone KJ, Fryer SM, Ryan T, Stoner L. The validity and reliability of continuous-wave near-infrared spectroscopy for the assessment of leg blood volume during an orthostatic challenge. Atherosclerosis. 2016; 251:234–239.
Article26. Lankford J, Numan M, Hashmi SS, Gourishankar A, Butler IJ. Cerebral blood flow during HUTT in young patients with orthostatic intolerance. Clin Auton Res. 2015; 25:277–284.
Article27. Perry BG, Cotter JD, Mejuto G, Mündel T, Lucas SJ. Cerebral hemodynamics during graded valsalva maneuvers. Front Physiol. 2014; 5:349.
Article28. Tsubaki A, Kojima S, Furusawa AA, Onishi H. Effect of valsalva maneuver-induced hemodynamic changes on brain near-infrared spectroscopy measurements. Adv Exp Med Biol. 2013; 789:97–103.
Article29. Saager R, Berger A. Measurement of layer-like hemodynamic trends in scalp and cortex: implications for physiological baseline suppression in functional near-infrared spectroscopy. J Biomed Opt. 2008; 13:034017.
Article30. Pott F, Van Lieshout JJ, Ide K, Madsen P, Secher NH. Middle cerebral artery blood velocity during intense static exercise is dominated by a valsalva maneuver. J Appl Physiol (1985). 2003; 94:1335–1344.
Article31. Laures M, Bolz N, Zhang Z, Mensen A, Schmidt C, Khatami R. Assessing the role of cerebral autoregulation during intrathoracic pressure changes by near infrared spectroscopy (NIRS). Eur Respir J. 2015; 46:PA2355.
Article32. Jones PK, Gibbons CH. Autonomic function testing: an important diagnostic test for patients with syncope. Pract Neurol. 2015; 15:346–351.
Article33. Assessment: clinical autonomic testing report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 1996; 46:873–880.34. Everdell NL, Airantzis D, Kolvya C, Suzuki T, Elwell CE. A portable wireless near-infrared spatially resolved spectroscopy system for use on brain and muscle. Med Eng Phys. 2013; 35:1692–1697.
Article35. Canova D, Roatta S, Bosone D, Micieli G. Inconsistent detection of changes in cerebral blood volume by near infrared spectroscopy in standard clinical tests. J Appl Physiol (1985). 2011; 110:1646–1655.
Article36. Szufladowicz E, Maniewski R, Kozluk E, Zbiec A, Nosek A, Walczak F. Near-infrared spectroscopy in evaluation of cerebral oxygenation during vasovagal syncope. Physiol Meas. 2004; 25:823–836.
Article37. Rao RP, Danduran MJ, Dixon JE, Frommelt PC, Berger S, Zangwill SD. Near infrared spectroscopy: guided tilt table testing for syncope. Pediatr Cardiol. 2010; 31:674–679.
Article38. Igarashi T, Sakatani K, Fujiwara N, Murata Y, Suma T, Shibuya T, et al. Monitoring of hemodynamic change in patients with carotid artery stenosis during the tilt test using wearable near-infrared spectroscopy. Adv Exp Med Biol. 2013; 789:463–467.
Article39. Damian MS, Schlosser R. Bilateral near infrared spectroscopy in space-occupying middle cerebral artery stroke. Neurocrit Care. 2007; 6:165–173.
Article40. Obrig H. NIRS in clinical neurology - a ‘promising’ tool? Neuroimage. 2014; 85(Pt 1):535–546.
Article41. Aries MJ, Coumou AD, Elting JW, van der Harst JJ, Kremer BP, Vroomen PC. Near infrared spectroscopy for the detection of desaturations in vulnerable ischemic brain tissue: a pilot study at the stroke unit bedside. Stroke. 2012; 43:1134–1136.
Article42. Pizza F, Biallas M, Kallweit U, Wolf M, Bassetti CL. Cerebral hemodynamic changes in stroke during sleep-disordered breathing. Stroke. 2012; 43:1951–1953.
Article43. Obrig H, Steinbrink J. Non-invasive optical imaging of stroke. Philos Trans A Math Phys Eng Sci. 2011; 369:4470–4494.
Article44. Sokol DK, Markand ON, Daly EC, Luerssen TG, Malkoff MD. Near infrared spectroscopy (NIRS) distinguishes seizure types. Seizure. 2000; 9:323–327.
Article45. Saito S, Yoshikawa D, Nishihara F, Morita T, Kitani Y, Amaya T, et al. The cerebral hemodynamic response to electrically induced seizures in man. Brain Res. 1995; 673:93–100.
Article46. Han CH, Song H, Kang YG, Kim BM, Im CH. Hemodynamic responses in rat brain during transcranial direct current stimulation: a functional near-infrared spectroscopy study. Biomed Opt Express. 2014; 5:1812–1821.
Article47. Byun JI, Jung KY, Lee GT, Kim CK, Kim BM. Spontaneous low-frequency cerebral hemodynamics oscillations in restless legs syndrome with periodic limb movements during sleep: a near-infrared spectroscopy study. J Clin Neurol. 2016; 12:107–114.
Article48. Nguyen DK, Tremblay J, Pouliot P, Vannasing P, Florea O, Carmant L, et al. Noninvasive continuous functional near-infrared spectroscopy combined with electroencephalography recording of frontal lobe seizures. Epilepsia. 2013; 54:331–340.
Article49. Watanabe E, Nagahori Y, Mayanagi Y. Focus diagnosis of epilepsy using near-infrared spectroscopy. Epilepsia. 2002; 43:Suppl 9. 50–55.
Article50. Lee S, Lee M, Koh D, Kim BM, Choi JH. Cerebral hemodynamic responses to seizure in the mouse brain: simultaneous near-infrared spectroscopy-electroencephalography study. J Biomed Opt. 2010; 15:037010.
Article51. Nguyen DK, Tremblay J, Pouliot P, Vannasing P, Florea O, Carmant L, et al. Non-invasive continuous EEG-fNIRS recording of temporal lobe seizures. Epilepsy Res. 2012; 99:112–126.
Article52. Liboni W, Molinari F, Chiribiri A, Allais G, Mana O, Negri E, et al. The diagnostic iter of patent foramen ovale in migraine patients: an update. Neurol Sci. 2008; 29:Suppl 1. S19–S22.
Article53. Liboni W, Molinari F, Allais GB, Mana O, Negri E, D'Andrea G, et al. Patent foramen ovale detected by near-infrared spectroscopy in patients suffering from migraine with aura. Neurol Sci. 2008; 29:Suppl 1. S182–S185.
Article54. Viola S, Viola P, Litterio P, Buongarzone MP, Fiorelli L. Pathophysiology of migraine attack with prolonged aura revealed by transcranial Doppler and near infrared spectroscopy. Neurol Sci. 2010; 31:Suppl 1. S165–S166.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Application of Near Infrared Spectroscopy
- Two-channel Near-infrared Spectroscopic Analysis of Association of Paranoia Symptoms with Prefrontal Activation
- Clinical Application of Near-Infrared Spectroscopy in Neonates
- Retraction: Application of near-infrared spectroscopy in clinical neurology
- Calculi in Hydrocele: Incidence and Results of Infrared Spectroscopy Analysis