Cancer Res Treat.
2019 Jul;51(3):1188-1197. 10.4143/crt.2018.434.
Impact of Mucin Proportion in the Pretreatment MRI on the Outcomes of Rectal Cancer Patients Undergoing Neoadjuvant Chemoradiotherapy
- Affiliations
-
- 1Department of Radiation Oncology, Seoul National University College of Medicine, Seoul, Korea. ekchie93@snu.ac.kr
- 2Department of Radiation Oncology, Korea Institute of Radiological & Medical Sciences, Seoul, Korea.
- 3Department of Radiation Oncology, Ewha Womans University College of Medicine, Seoul, Korea. kyubokim.ro@gmail.com
- 4Department of Radiology, Seoul National University College of Medicine, Seoul, Korea.
- 5Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 6Department of Surgery, Seoul National University College of Medicine, Seoul, Korea.
- 7Department of Pathology, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2454310
- DOI: http://doi.org/10.4143/crt.2018.434
Abstract
- PURPOSE
The purpose of this study was to evaluate treatment response to neoadjuvant chemoradiotherapy (CRT) with regard to mucin status in pathology and pretreatment magnetic resonance imaging (MRI) in locally advanced rectal cancer.
MATERIALS AND METHODS
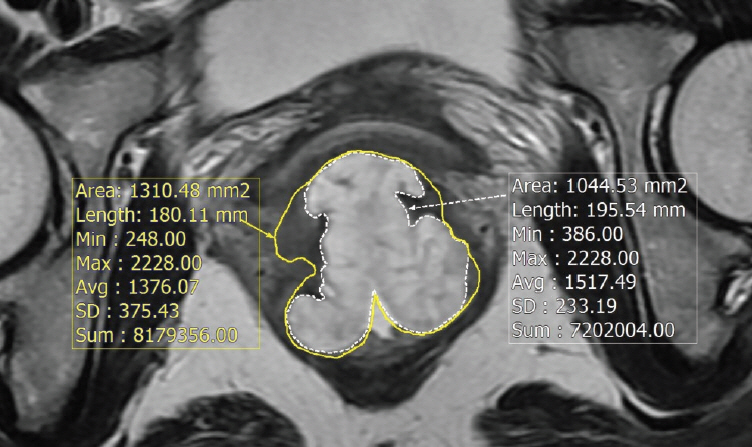
Between 2003 and 2011, 306 patients with locally advanced rectal cancer received neoadjuvant CRT followed by surgery, and mucinous adenocarcinoma (MAC) was found in 27 (8.8%). All MAC patients had MRI before and after CRT and mucin proportion at MRI was measured. Therapeutic response was assessed by pathology after total mesorectal excision. To determine the optimal cut-off for mucin proportion in predicting good CRT response (near total or total regression) and negative circumferential resection margin (CRM), the receiver-operating characteristic analysis was performed.
RESULTS
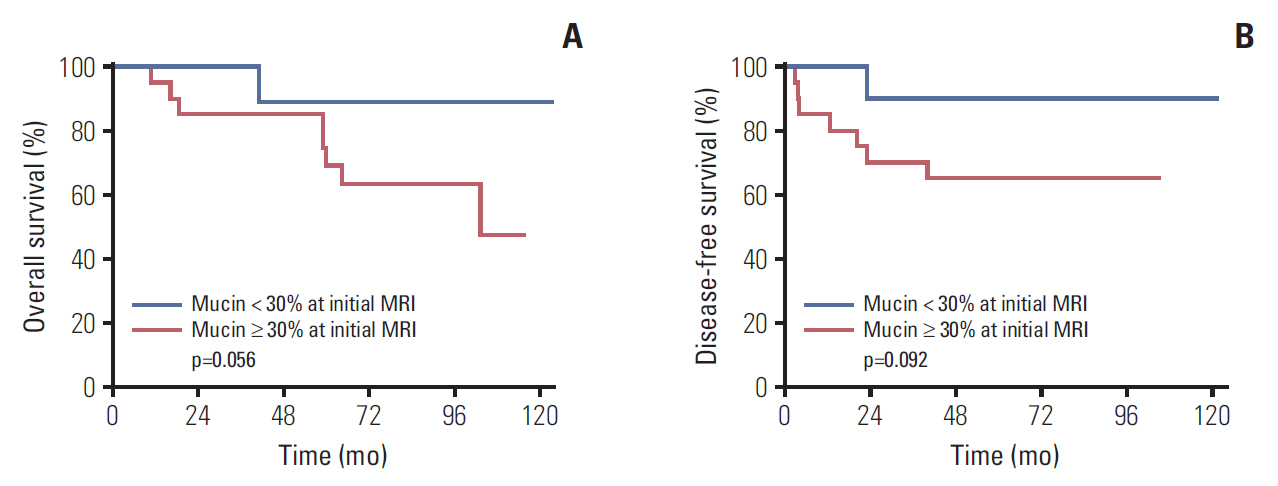
After neoadjuvant CRT, overall downstaging occurred in 44.4% of MAC and 72.4% of non-MAC (p=0.001), and positive CRM (≤1 mm) was observed more frequently in MAC (p<0.001). The optimal threshold for treatment response was 30% for mucin proportion, and there are nine with low mucin proportion (<30%) and 18 with high mucin proportion (≥30%) in pretreatment MRI. Negative CRM and tumor downstaging occurred more common in patients with mucin <30%, although statistically insignificant (p=0.071 and p=0.072, respectively). Regarding oncologic outcomes, lower mucin proportion in pretreatment MRI was associated with better disease-free and overall survival in MAC group (p=0.092 and 0.056, respectively), but the difference did not reach statistical significance.
CONCLUSION
Poor treatment outcome with neoadjuvant CRT was observed in patients with MAC, especially those with high mucin proportion at pretreatment MRI.
MeSH Terms
Figure
Reference
-
References
1. Compton CC, Fielding LP, Burgart LJ, Conley B, Cooper HS, Hamilton SR, et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000; 124:979–94.2. Verhulst J, Ferdinande L, Demetter P, Ceelen W. Mucinous subtype as prognostic factor in colorectal cancer: a systematic review and meta-analysis. J Clin Pathol. 2012; 65:381–8.
Article3. Hanski C. Is mucinous carcinoma of the colorectum a distinct genetic entity? Br J Cancer. 1995; 72:1350–6.
Article4. Halvorsen TB, Seim E. Influence of mucinous components on survival in colorectal adenocarcinomas: a multivariate analysis. J Clin Pathol. 1988; 41:1068–72.
Article5. Hermanek P, Guggenmoos-Holzmann I, Gall FP. Prognostic factors in rectal carcinoma: a contribution to the further development of tumor classification. Dis Colon Rectum. 1989; 32:593–9.6. Hugen N, van de Velde CJ, Bosch SL, Futterer JJ, Elferink MA, Marijnen CA, et al. Modern treatment of rectal cancer closes the gap between common adenocarcinoma and mucinous carcinoma. Ann Surg Oncol. 2015; 22:2669–76.
Article7. Simha V, Kapoor R, Gupta R, Bahl A, Nada R. Mucinous adenocarcinoma of the rectum: a poor candidate for neo-adjuvant chemoradiation? J Gastrointest Oncol. 2014; 5:276–9.8. Shin US, Yu CS, Kim JH, Kim TW, Lim SB, Yoon SN, et al. Mucinous rectal cancer: effectiveness of preoperative chemoradiotherapy and prognosis. Ann Surg Oncol. 2011; 18:2232–9.
Article9. Grillo-Ruggieri F, Mantello G, Berardi R, Cardinali M, Fenu F, Iovini G, et al. Mucinous rectal adenocarcinoma can be associated to tumor downstaging after preoperative chemoradiotherapy. Dis Colon Rectum. 2007; 50:1594–603.
Article10. Kim MJ, Park JS, Park SI, Kim NK, Kim JH, Moon HJ, et al. Accuracy in differentiation of mucinous and nonmucinous rectal carcinoma on MR imaging. J Comput Assist Tomogr. 2003; 27:48–55.
Article11. Oberholzer K, Menig M, Kreft A, Schneider A, Junginger T, Heintz A, et al. Rectal cancer: mucinous carcinoma on magnetic resonance imaging indicates poor response to neoadjuvant chemoradiation. Int J Radiat Oncol Biol Phys. 2012; 82:842–8.
Article12. Chang HJ, Park CK, Kim WH, Kim YB, Kim YW, Kim HG, et al. A standardized pathology report for colorectal cancer. Korean J Pathol. 2006; 40:193–203.13. McCawley N, Clancy C, O'Neill BD, Deasy J, McNamara DA, Burke JP. Mucinous rectal adenocarcinoma is associated with a poor response to neoadjuvant chemoradiotherapy: a systematic review and meta-analysis. Dis Colon Rectum. 2016; 59:1200–8.
Article14. Hyngstrom JR, Hu CY, Xing Y, You YN, Feig BW, Skibber JM, et al. Clinicopathology and outcomes for mucinous and signet ring colorectal adenocarcinoma: analysis from the National Cancer Data Base. Ann Surg Oncol. 2012; 19:2814–21.
Article15. Hugen N, Verhoeven RH, Radema SA, de Hingh IH, Pruijt JF, Nagtegaal ID, et al. Prognosis and value of adjuvant chemotherapy in stage III mucinous colorectal carcinoma. Ann Oncol. 2013; 24:2819–24.
Article16. Hogan J, Burke JP, Samaha G, Condon E, Waldron D, Faul P, et al. Overall survival is improved in mucinous adenocarcinoma of the colon. Int J Colorectal Dis. 2014; 29:563–9.
Article17. Negri FV, Wotherspoon A, Cunningham D, Norman AR, Chong G, Ross PJ. Mucinous histology predicts for reduced fluorouracil responsiveness and survival in advanced colorectal cancer. Ann Oncol. 2005; 16:1305–10.
Article18. Catalano V, Loupakis F, Graziano F, Torresi U, Bisonni R, Mari D, et al. Mucinous histology predicts for poor response rate and overall survival of patients with colorectal cancer and treated with first-line oxaliplatin- and/or irinotecan-based chemotherapy. Br J Cancer. 2009; 100:881–7.
Article19. Kazama Y, Watanabe T, Kanazawa T, Tada T, Tanaka J, Nagawa H. Mucinous carcinomas of the colon and rectum show higher rates of microsatellite instability and lower rates of chromosomal instability: a study matched for T classification and tumor location. Cancer. 2005; 103:2023–9.20. Carethers JM, Chauhan DP, Fink D, Nebel S, Bresalier RS, Howell SB, et al. Mismatch repair proficiency and in vitro response to 5-fluorouracil. Gastroenterology. 1999; 117:123–31.
Article21. Jover R, Zapater P, Castells A, Llor X, Andreu M, Cubiella J, et al. Mismatch repair status in the prediction of benefit from adjuvant fluorouracil chemotherapy in colorectal cancer. Gut. 2006; 55:848–55.
Article22. Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003; 349:247–57.
Article23. Dolcetti R, Viel A, Doglioni C, Russo A, Guidoboni M, Capozzi E, et al. High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am J Pathol. 1999; 154:1805–13.
Article24. Xiao Y, Freeman GJ. The microsatellite instable subset of colorectal cancer is a particularly good candidate for checkpoint blockade immunotherapy. Cancer Discov. 2015; 5:16–8.
Article25. Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015; 5:43–51.
Article26. Friedman EJ. Immune modulation by ionizing radiation and its implications for cancer immunotherapy. Curr Pharm Des. 2002; 8:1765–80.
Article27. Compton CC. Key issues in reporting common cancer specimens: problems in pathologic staging of colon cancer. Arch Pathol Lab Med. 2006; 130:318–24.
Article28. Shia J, McManus M, Guillem JG, Leibold T, Zhou Q, Tang LH, et al. Significance of acellular mucin pools in rectal carcinoma after neoadjuvant chemoradiotherapy. Am J Surg Pathol. 2011; 35:127–34.
Article29. Hussain SM, Outwater EK, Siegelman ES. Mucinous versus nonmucinous rectal carcinomas: differentiation with MR imaging. Radiology. 1999; 213:79–85.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Negative impact of pretreatment anemia on local control after neoadjuvant chemoradiotherapy and surgery for rectal cancer
- Lateral Pelvic Lymph Node Dissection After Neoadjuvant Chemoradiotherapy in Patients With Rectal Cancer: A Single-Center Experience and Literature Review
- Correlation between tumor regression grade and rectal volume in neoadjuvant concurrent chemoradiotherapy for rectal cancer
- Clinical characteristics of rectal cancer patients with neoadjuvant chemoradiotherapy: a nationwide population-based cohort study in South Korea
- Interpretation of Rectal MRI after Neoadjuvant Treatment in Patients with Rectal Cancer