Allergy Asthma Immunol Res.
2019 Sep;11(5):677-690. 10.4168/aair.2019.11.5.677.
Activated Leukocyte Cell Adhesion Molecule Modulates Th2 Immune Response in Atopic Dermatitis
- Affiliations
-
- 1Department of Pediatrics, Severance Hospital, Institute of Allergy, Institute for Immunology and Immunological Diseases, Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea. mhsohn@yuhs.ac
- 2Department of Pediatrics, CHA Bundang Medical Center, CHA University School of Medicine, Seongnam, Korea.
- 3Department of Dermatology and Cutaneous Biology Research Institute, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2452758
- DOI: http://doi.org/10.4168/aair.2019.11.5.677
Abstract
- PURPOSE
Activated leukocyte cell adhesion molecule (ALCAM), a member of the immunoglobulin superfamily, is highly expressed on dendritic cells. ALCAM and its receptor CD6 are co-stimulatory molecules in the immunological synapse; their interaction is required for T cell activation. While atopic dermatitis (AD) is recognized as a T helper 2 (Th2)-mediated allergic disease, the role of ALCAM in its pathogenesis is unclear.
METHODS
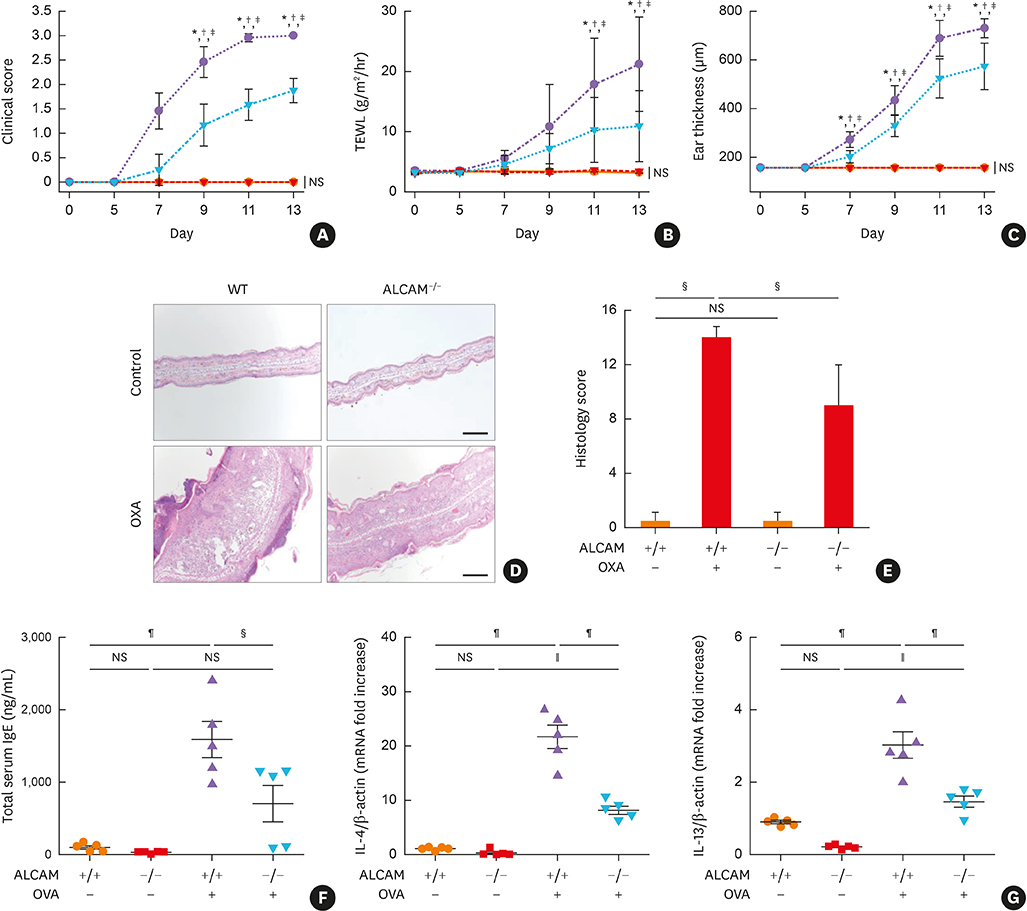
ALCAM levels were measured in the serum of AD patients and AD-induced murine model by ovalbumin treatment. We next investigated transepidermal water loss, clinical score, Th2-immune responses, skin barrier gene expression and T-cell activation using wild-type (WT) and ALCAM deficiency mice. An oxazolone-induced AD-like model was also established and analyzed using WT- and ALCAM-deficient mice.
RESULTS
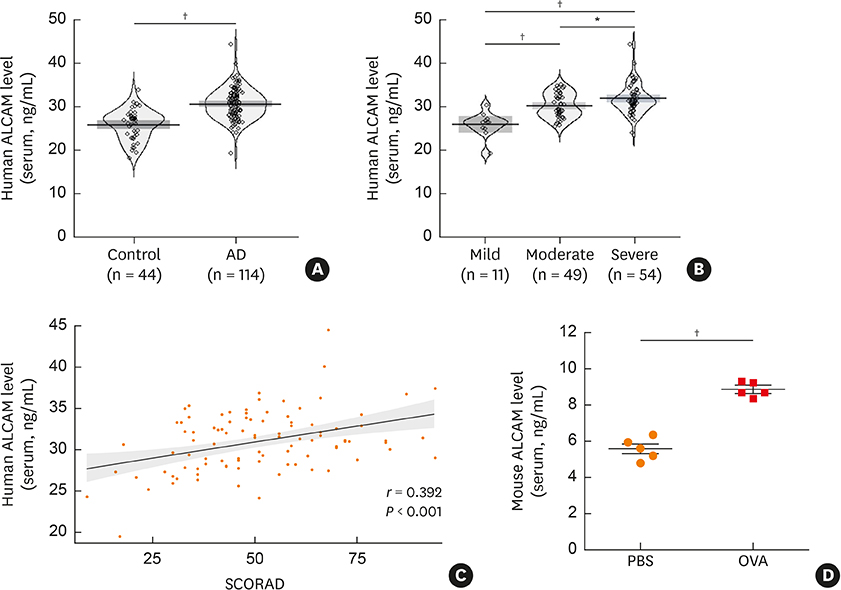
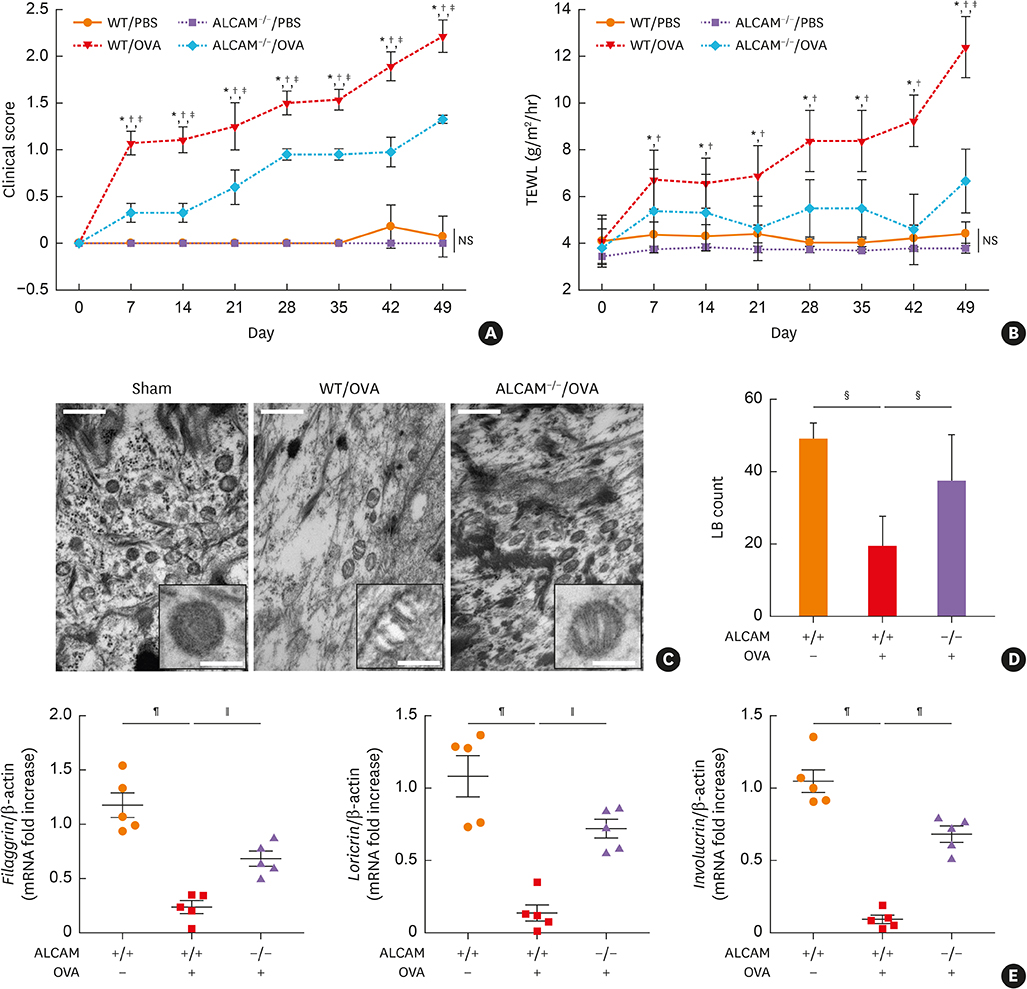
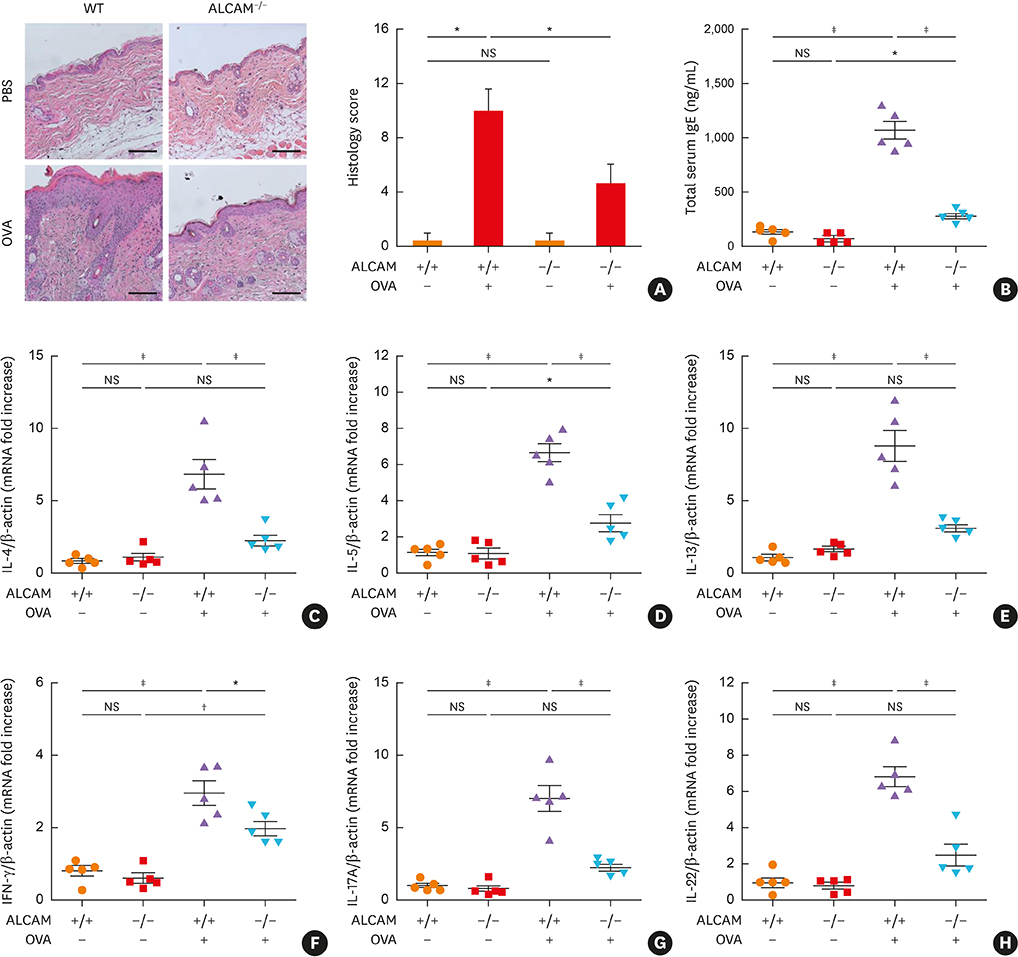
We found that serum ALCAM levels were elevated in pediatric AD patients as well as WT AD mice, whereas Th2-type cytokine production and AD symptoms were suppressed in ALCAM-deficient mice. In addition, CD4+ effector T-cell counts in murine skin and skin-draining lymph nodes were lower in ALCAM-deficient mice than in their WT counterparts. ALCAM deficiency was also linked to higher expression of skin barrier genes and number of lamellar bodies.
CONCLUSIONS
These findings indicate that ALCAM may contribute to AD pathogenesis by meditating a Th2-dominant immune response and disrupting the barrier function of the skin.
Keyword
MeSH Terms
Figure
Reference
-
1. Yanaba K, Kamata M, Asano Y, Tada Y, Sugaya M, Kadono T, et al. CD19 expression in B cells regulates atopic dermatitis in a mouse model. Am J Pathol. 2013; 182:2214–2222.
Article2. Di Cesare A, Di Meglio P, Nestle FO. A role for Th17 cells in the immunopathogenesis of atopic dermatitis? J Invest Dermatol. 2008; 128:2569–2571.3. Peng W, Novak N. Pathogenesis of atopic dermatitis. Clin Exp Allergy. 2015; 45:566–574.
Article4. Pyun BY. Natural history and risk factors of atopic dermatitis in children. Allergy Asthma Immunol Res. 2015; 7:101–105.
Article5. Han DK, Kim MK, Yoo JE, Choi SY, Kwon BC, Sohn MH, et al. Food sensitization in infants and young children with atopic dermatitis. Yonsei Med J. 2004; 45:803–809.
Article6. Venturelli N, Lexmond WS, Ohsaki A, Nurko S, Karasuyama H, Fiebiger E, et al. Allergic skin sensitization promotes eosinophilic esophagitis through the IL-33-basophil axis in mice. J Allergy Clin Immunol. 2016; 138:1367–1380.e5.
Article7. Galand C, Leyva-Castillo JM, Yoon J, Han A, Lee MS, McKenzie AN, et al. IL-33 promotes food anaphylaxis in epicutaneously sensitized mice by targeting mast cells. J Allergy Clin Immunol. 2016; 138:1356–1366.
Article8. Dhingra N, Gulati N, Guttman-Yassky E. Mechanisms of contact sensitization offer insights into the role of barrier defects vs. intrinsic immune abnormalities as drivers of atopic dermatitis. J Invest Dermatol. 2013; 133:2311–2314.
Article9. Leung DY, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. J Clin Invest. 2004; 113:651–657.
Article10. Mamessier E, Magnan A. Cytokines in atopic diseases: revisiting the Th2 dogma. Eur J Dermatol. 2006; 16:103–113.11. Heeringa JJ, Fieten KB, Bruins FM, van Hoffen E, Knol EF, Pasmans SG, et al. Treatment for moderate to severe atopic dermatitis in alpine and moderate maritime climates differentially affects helper T cells and memory B cells in children. Clin Exp Allergy. 2018; 48:679–690.
Article12. Hennino A, Vocanson M, Toussaint Y, Rodet K, Benetière J, Schmitt AM, et al. Skin-infiltrating CD8+ T cells initiate atopic dermatitis lesions. J Immunol. 2007; 178:5571–5577.
Article13. van der Heijden FL, Wierenga EA, Bos JD, Kapsenberg ML. High frequency of IL-4-producing CD4+ allergen-specific T lymphocytes in atopic dermatitis lesional skin. J Invest Dermatol. 1991; 97:389–394.
Article14. Otsuka A, Nomura T, Rerknimitr P, Seidel JA, Honda T, Kabashima K. The interplay between genetic and environmental factors in the pathogenesis of atopic dermatitis. Immunol Rev. 2017; 278:246–262.
Article15. Vandeghinste N, Klattig J, Jagerschmidt C, Lavazais S, Marsais F, Haas JD, et al. Neutralization of IL-17C reduces skin inflammation in mouse models of psoriasis and atopic dermatitis. J Invest Dermatol. 2018; 138:1555–1563.
Article16. Heo WI, Lee KE, Hong JY, Kim MN, Oh MS, Kim YS, et al. The role of interleukin-17 in mouse models of atopic dermatitis and contact dermatitis. Clin Exp Dermatol. 2015; 40:665–671.
Article17. Fuhlbrigge RC, King SL, Sackstein R, Kupper TS. CD43 is a ligand for E-selectin on CLA+ human T cells. Blood. 2006; 107:1421–1426.
Article18. Lee JH, Lee YS, Lee EJ, Lee JH, Kim TY. Capsiate inhibits DNFB-induced atopic dermatitis in NC/Nga mice through mast cell and CD4+ T-cell inactivation. J Invest Dermatol. 2015; 135:1977–1985.
Article19. Fesenkova VI, Kurchenko AI, Castellani ML, Conti P, Anogeianaki A, Caraffa A, et al. Expression of co-stimulatory molecules on langerhans cells in lesional epidermis of human atopic dermatitis. Immunopharmacol Immunotoxicol. 2007; 29:487–498.
Article20. Gutcher I, Becher B. APC-derived cytokines and T cell polarization in autoimmune inflammation. J Clin Invest. 2007; 117:1119–1127.
Article21. Donizy P, Zietek M, Halon A, Leskiewicz M, Kozyra C, Matkowski R. Prognostic significance of ALCAM (CD166/MEMD) expression in cutaneous melanoma patients. Diagn Pathol. 2015; 10:86.
Article22. Swart GW. Activated leukocyte cell adhesion molecule (CD166/ALCAM): developmental and mechanistic aspects of cell clustering and cell migration. Eur J Cell Biol. 2002; 81:313–321.
Article23. Zimmerman AW, Joosten B, Torensma R, Parnes JR, van Leeuwen FN, Figdor CG. Long-term engagement of CD6 and ALCAM is essential for T-cell proliferation induced by dendritic cells. Blood. 2006; 107:3212–3220.
Article24. Weidle UH, Eggle D, Klostermann S, Swart GW. ALCAM/CD166: cancer-related issues. Cancer Genomics Proteomics. 2010; 7:231–243.25. Hassan NJ, Simmonds SJ, Clarkson NG, Hanrahan S, Puklavec MJ, Bomb M, et al. CD6 regulates T-cell responses through activation-dependent recruitment of the positive regulator SLP-76. Mol Cell Biol. 2006; 26:6727–6738.
Article26. Islam SA, Luster AD. T cell homing to epithelial barriers in allergic disease. Nat Med. 2012; 18:705–715.
Article27. Kato Y, Tanaka Y, Hayashi M, Okawa K, Minato N. Involvement of CD166 in the activation of human gamma delta T cells by tumor cells sensitized with nonpeptide antigens. J Immunol. 2006; 177:877–884.28. Kim MN, Hong JY, Shim DH, Sol IS, Kim YS, Lee JH, et al. Activated leukocyte cell adhesion molecule stimulates the T-cell response in allergic asthma. Am J Respir Crit Care Med. 2018; 197:994–1008.
Article29. Kim YS, Kim MN, Lee KE, Hong JY, Oh MS, Kim SY, et al. Activated leucocyte cell adhesion molecule (ALCAM/CD166) regulates T cell responses in a murine model of food allergy. Clin Exp Immunol. 2018; 192:151–164.
Article30. Eichenfield LF, Hanifin JM, Luger TA, Stevens SR, Pride HB. Consensus conference on pediatric atopic dermatitis. J Am Acad Dermatol. 2003; 49:1088–1095.
Article31. Severity scoring of atopic dermatitis: the SCORAD index. Consensus report of the European task force on atopic dermatitis. Dermatology. 1993; 186:23–31.32. Oranje AP, Glazenburg EJ, Wolkerstorfer A, de Waard-van der Spek FB. Practical issues on interpretation of scoring atopic dermatitis: the SCORAD index, objective SCORAD and the three-item severity score. Br J Dermatol. 2007; 157:645–648.
Article33. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1980; 92:44–47.34. Jin H, He R, Oyoshi M, Geha RS. Animal models of atopic dermatitis. J Invest Dermatol. 2009; 129:31–40.
Article35. Moniaga CS, Jeong SK, Egawa G, Nakajima S, Hara-Chikuma M, Jeon JE, et al. Protease activity enhances production of thymic stromal lymphopoietin and basophil accumulation in flaky tail mice. Am J Pathol. 2013; 182:841–851.
Article36. Almeida FF, Tenno M, Brzostek J, Li JL, Allies G, Hoeffel G, et al. Identification of a novel lymphoid population in the murine epidermis. Sci Rep. 2015; 5:12554.
Article37. Elias PM, Hatano Y, Williams ML. Basis for the barrier abnormality in atopic dermatitis: outside-inside-outside pathogenic mechanisms. J Allergy Clin Immunol. 2008; 121:1337–1343.
Article38. Krishnan L, Gurnani K, Dicaire CJ, van Faassen H, Zafer A, Kirschning CJ, et al. Rapid clonal expansion and prolonged maintenance of memory CD8+ T cells of the effector (CD44highCD62Llow) and central (CD44highCD62Lhigh) phenotype by an archaeosome adjuvant independent of TLR2. J Immunol. 2007; 178:2396–2406.39. van Kempen LC, van den Oord JJ, van Muijen GN, Weidle UH, Bloemers HP, Swart GW. Activated leukocyte cell adhesion molecule/CD166, a marker of tumor progression in primary malignant melanoma of the skin. Am J Pathol. 2000; 156:769–774.
Article40. von Bauer R, Oikonomou D, Sulaj A, Mohammed S, Hotz-Wagenblatt A, Gröne HJ, et al. CD166/ALCAM mediates proinflammatory effects of S100B in delayed type hypersensitivity. J Immunol. 2013; 191:369–377.
Article41. Ibáñez A, Sarrias MR, Farnós M, Gimferrer I, Serra-Pagès C, Vives J, et al. Mitogen-activated protein kinase pathway activation by the CD6 lymphocyte surface receptor. J Immunol. 2006; 177:1152–1159.
Article42. Kim BE, Leung DY, Boguniewicz M, Howell MD. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin Immunol. 2008; 126:332–337.
Article43. Sehra S, Yao Y, Howell MD, Nguyen ET, Kansas GS, Leung DY, et al. IL-4 regulates skin homeostasis and the predisposition toward allergic skin inflammation. J Immunol. 2010; 184:3186–3190.
Article44. Elias PM, Wakefield JS. Mechanisms of abnormal lamellar body secretion and the dysfunctional skin barrier in patients with atopic dermatitis. J Allergy Clin Immunol. 2014; 134:781–791.e1.
Article45. Hönzke S, Wallmeyer L, Ostrowski A, Radbruch M, Mundhenk L, Schäfer-Korting M, et al. Influence of Th2 cytokines on the cornified envelope, tight junction proteins, and ß-defensins in filaggrin-deficient skin equivalents. J Invest Dermatol. 2016; 136:631–639.
Article46. Bughani U, Saha A, Kuriakose A, Nair R, Sadashivarao RB, Venkataraman R, et al. T cell activation and differentiation is modulated by a CD6 domain 1 antibody Itolizumab. PLoS ONE. 2017; 12:e0180088.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Therapeutic effect of Dermatophagoides farinae antigen - autoantibody immune complex therapy in atopic dermatitis
- Effects of probiotics on the prevention of atopic dermatitis
- Inhibition of Leukocyte Adhesion by Developmental Endothelial Locus-1 (Del-1)
- Expression of cell adhesion molecules on positive reaction site of patch test with Dermatophagoides farinae in atopic dermatitis patients
- Two Cases of Atopic Dermatitis Developing Ocular Complication and Immunological Disturbance