Anat Cell Biol.
2019 Jun;52(2):183-190. 10.5115/acb.2019.52.2.183.
Genistein improve nicotine toxicity on male mice pancreas
- Affiliations
-
- 1Department of Anatomical Sciences, Medical School, Kermanshah University of Medical Sciences, Kermanshah, Iran.
- 2Department of Anatomical Sciences, Medical School, Hamedan University of Medical Sciences, Hamedan, Iran.
- 3Students Research Committee, Kermanshah University of Medical Sciences, Kermanshah, Iran.
- 4Medical Biology Research Center, Kermanshah University of Medical Sciences, Kermanshah, Iran. cjalili@yahoo.com
- KMID: 2451222
- DOI: http://doi.org/10.5115/acb.2019.52.2.183
Abstract
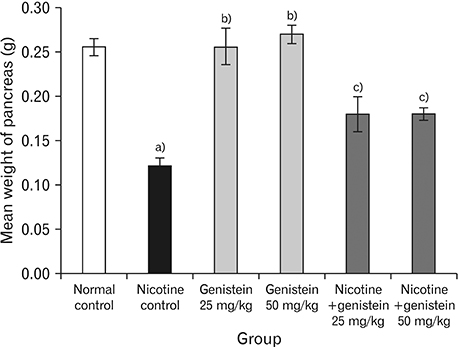
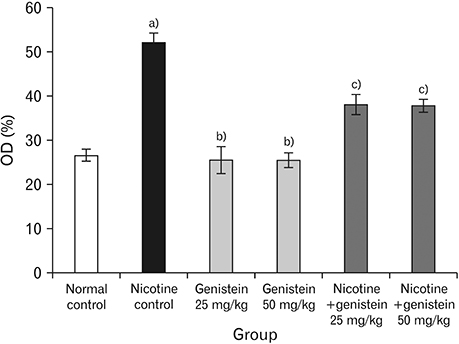
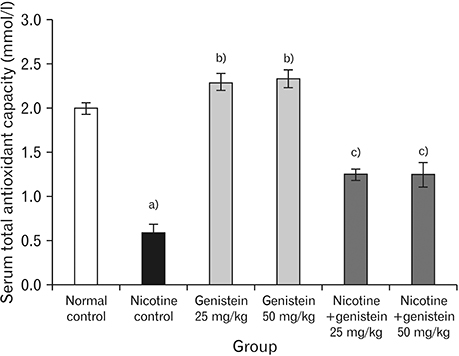
- Nicotine is the most toxic factor of tobacco. Genistein is a phytoestrogen and antioxidant that has numerous health benefits. The aim of this study is to evaluate the effects of genistein against toxic properties of nicotine to the pancreas of mice. For this purpose, 48 male mice were randomly assigned into six groups (n=8): normal control, nicotine control (2.5 mg/kg), genistein (25 and 50 mg/kg), and nicotine+genistein (25 and 50 mg/kg) treated groups. Various doses of genistein and genistein+nicotine were administered intraperitoneally to animals for 4 weeks. The weight of pancreas, total antioxidant capacity and nitrite oxide of serum, insulin levels, and the number and diameter of islets of Langerhans were investigated. Nicotine administration reduced significantly total antioxidant capacity, insulin, pancreas weight, and the number and diameter of islets of Langerhans and increased nitrite oxide in serum compared to the control normal group (P<0.05). Conversely, genistein and genistein+nicotine increased significantly insulin, total antioxidant capacity, and the number and diameter islets of Langerhans and decreased serum nitrite oxide compared to the nicotine control group. It seems that the genistein can improve pancreas damage following the nicotine administration.
MeSH Terms
Figure
Cited by 1 articles
-
Nicotine impact on rat substantia nigra compacta
Sanaa A M Elgayar, Ola A Hussein, Heba A Mubarak, Amany M Ismaiel, Asmaa M.S. Gomaa
Anat Cell Biol. 2021;54(1):112-123. doi: 10.5115/acb.20.267.
Reference
-
1. Salahshoor MR, Roshankhah S, Hosseni P, Jalili C. Genistein improves liver damage in male mice exposed to morphine. Chin Med J (Engl). 2018; 131:1598–1604.
Article2. Velasquez MT, Bhathena SJ. Role of dietary soy protein in obesity. Int J Med Sci. 2007; 4:72–82.
Article3. Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect. 2006; 114:567–572.4. Pavese JM, Farmer RL, Bergan RC. Inhibition of cancer cell invasion and metastasis by genistein. Cancer Metastasis Rev. 2010; 29:465–482.
Article5. Lee JS. Effects of soy protein and genistein on blood glucose, antioxidant enzyme activities, and lipid profile in streptozotocin-induced diabetic rats. Life Sci. 2006; 79:1578–1584.
Article6. Behloul N, Wu G. Genistein: a promising therapeutic agent for obesity and diabetes treatment. Eur J Pharmacol. 2013; 698:31–38.
Article7. Gawish AM, Ramadan S, Hassan AM, Issa AM. Morphometrical, histopathological, and cytogenetical ameliorating effects of green tea extract on nicotine toxicity of the testis of rats. J Cytol Histol. 2010; 1:1105.
Article8. Panahi S, Abdollahifar MA, Aliaghaei A, Nazarian H, Paktinat S, Abdi S, Farahani RM. Application of stereological methods for unbiased estimation of sperm morphology in the mice induced by busulfan. Anat Cell Biol. 2017; 50:301–305.
Article9. Kadiyala V, Lee LS, Banks PA, Suleiman S, Paulo JA, Wang W, Rosenblum J, Sainani NI, Mortele K, Conwell DL. Cigarette smoking impairs pancreatic duct cell bicarbonate secretion. JOP. 2013; 14:31–38.10. Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003; 421:384–388.11. Polkowski K, Mazurek AP. Biological properties of genistein. A review of in vitro and in vivo data. Acta Pol Pharm. 2000; 57:135–155.12. Jalili C, Salahshoor MR, Hoseini M, Roshankhah S, Sohrabi M, Shabanizadeh A. Protective effect of thymoquinone against morphine injuries to kidneys of mice. Iran J Kidney Dis. 2017; 11:142–150.13. Wan C, Jin F, Du Y, Yang K, Yao L, Mei Z, Huang W. Genistein improves schistosomiasis liver granuloma and fibrosis via dampening NF-kB signaling in mice. Parasitol Res. 2017; 116:1165–1174.
Article14. Neuman JC, Truchan NA, Joseph JW, Kimple ME. A method for mouse pancreatic islet isolation and intracellular cAMP determination. J Vis Exp. 2014; e50374.
Article15. Elayat AA, el-Naggar MM, Tahir M. An immunocytochemical and morphometric study of the rat pancreatic islets. J Anat. 1995; 186(Pt 3):629–637.16. Borai A, Livingstone C, Kaddam I, Ferns G. Selection of the appropriate method for the assessment of insulin resistance. BMC Med Res Methodol. 2011; 11:158.
Article17. Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996; 239:70–76.
Article18. Thrower E. Pathologic cellular events in smoking-related pancreatitis. Cancers (Basel). 2015; 7:723–735.
Article19. Huang H, Xu Y, van den Pol AN. Nicotine excites hypothalamic arcuate anorexigenic proopiomelanocortin neurons and orexigenic neuropeptide Y neurons: similarities and differences. J Neurophysiol. 2011; 106:1191–1202.
Article20. Mineur YS, Abizaid A, Rao Y, Salas R, DiLeone RJ, Gundisch D, Diano S, De Biasi M, Horvath TL, Gao XB, Picciotto MR. Nicotine decreases food intake through activation of POMC neurons. Science. 2011; 332:1330–1332.
Article21. Williams DL, Schwartz MW. The melanocortin system as a central integrator of direct and indirect controls of food intake. Am J Physiol Regul Integr Comp Physiol. 2005; 289:R2–R3.
Article22. Tamm C, Zhivotovsky B, Ceccatelli S. Caspase-2 activation in neural stem cells undergoing oxidative stress-induced apoptosis. Apoptosis. 2008; 13:354–363.
Article23. Hashemi J, Pasalar P, Soleimani M, Khorramirouz R, Fendereski K, Enderami SE, Kajbafzadeh AM. Application of a novel bioreactor for in vivo engineering of pancreas tissue. J Cell Physiol. 2018; 233:3805–3816.24. Scuro LS, Simioni PU, Grabriel DL, Saviani EE, Modolo LV, Tamashiro WM, Salgado I. Suppression of nitric oxide production in mouse macrophages by soybean flavonoids accumulated in response to nitroprusside and fungal elicitation. BMC Biochem. 2004; 5:5.25. Guo TL, Germolec DR, Zheng JF, Kooistra L, Auttachoat W, Smith MJ, White KL, Elmore SA. Genistein protects female nonobese diabetic mice from developing type 1 diabetes when fed a soy- and alfalfa-free diet. Toxicol Pathol. 2015; 43:435–448.
Article26. Jalili C, Ahmadi S, Roshankhah S, Salahshoor M. Effect of Genistein on reproductive parameter and serum nitric oxide levels in morphine-treated mice. Int J Reprod Biomed (Yazd). 2016; 14:95–102.
Article27. Gumustekin K, Ciftci M, Coban A, Altikat S, Aktas O, Gul M, Timur H, Dane S. Effects of nicotine and vitamin E on glucose 6-phosphate dehydrogenase activity in some rat tissues in vivo and in vitro. J Enzyme Inhib Med Chem. 2005; 20:497–502.28. Fu Z, Zhang W, Zhen W, Lum H, Nadler J, Bassaganya-Riera J, Jia Z, Wang Y, Misra H, Liu D. Genistein induces pancreatic beta-cell proliferation through activation of multiple signaling pathways and prevents insulin-deficient diabetes in mice. Endocrinology. 2010; 151:3026–3037.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Experimental Studies on the Relation between Nicotine and Sexual Hormone: Part IV. The Antidotal Action of Luteohormone on Nicotine Toxicity during Anaphylaxis: C. The Effect of Nicotine with Luteohormone on Anaphylaxis
- Experimental Studies on the Relation between Nicotine and Sexual Hormone: Part IV. The Antidotal Action of Luteohormone on Nicotine Toxicity during Anaphylaxis: B. The Effect of Luteohormone on Anaphylaxis
- Experimental Studies on the Relation between Nicotine and Sexual Hormone: Part IV. The Antidotal Action of Luteohormone on Nicotine Toxicity during Anaphylaxis: A. The Effect of Nicotine on Blood Sugar, Blood Pressure and Anaphylaxis
- Experimental Studies on the Relation between Nicotine and Sexual Hormone: Part II. Effect of Castration and Sexual Hormone on Nicotine Toxicity
- Genistein Improves the Major Depression through Suppressing the Expression of miR-221/222 by Targeting Connexin 43