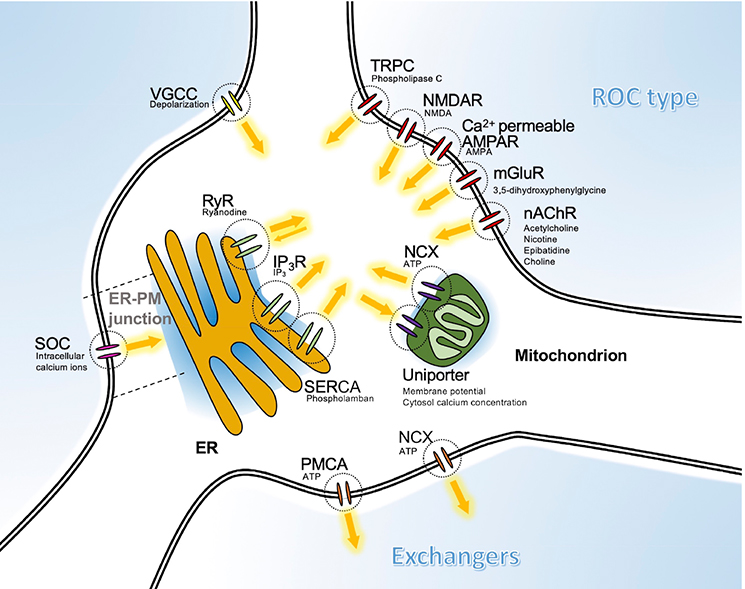
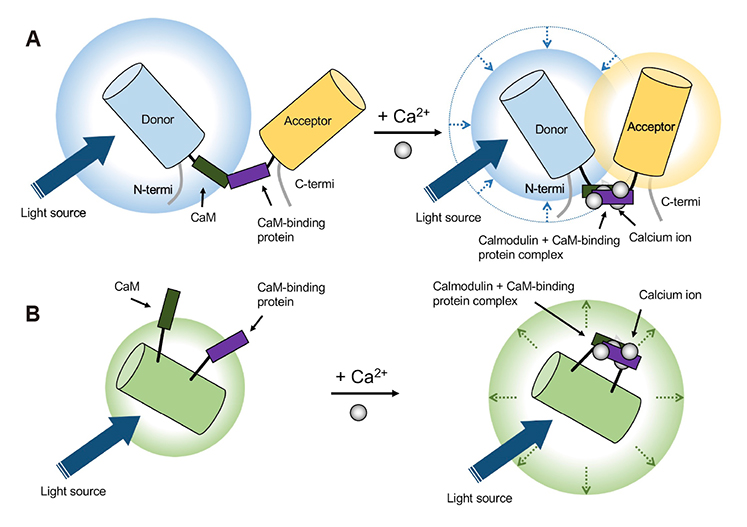
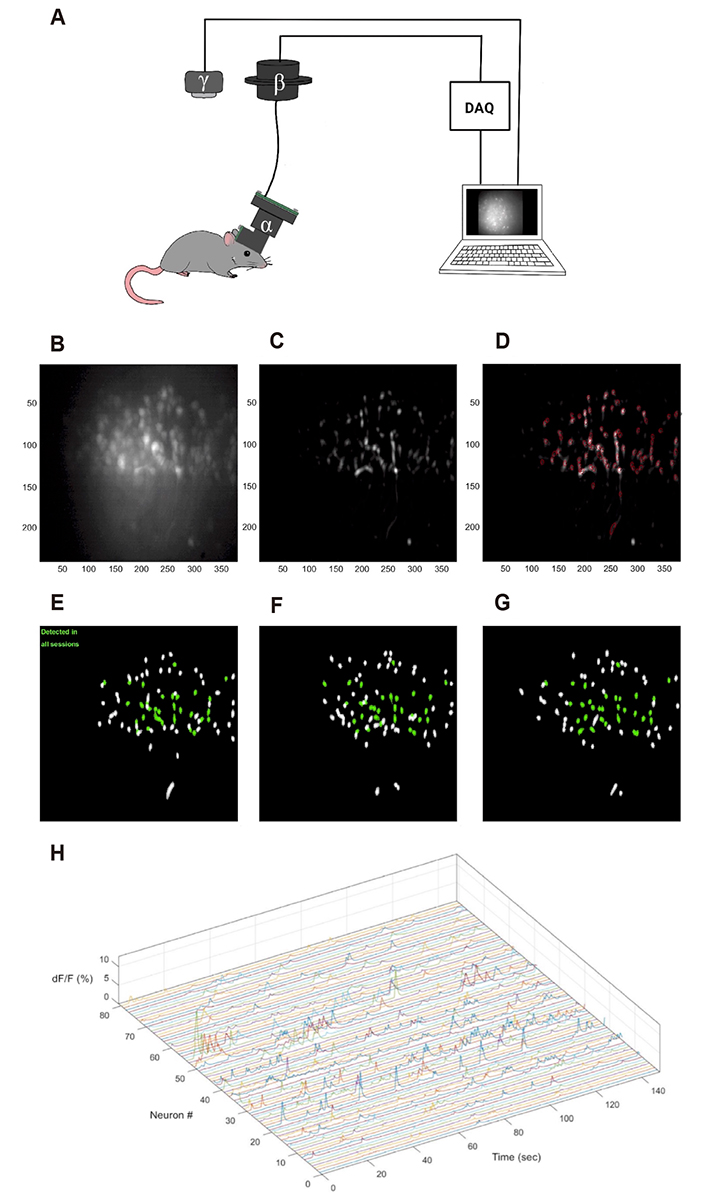
Korean J Physiol Pharmacol.
2019 Jul;23(4):237-249. 10.4196/kjpp.2019.23.4.237.
Imaging and analysis of genetically encoded calcium indicators linking neural circuits and behaviors
- Affiliations
-
- 1School of Biological Sciences, Seoul National University, Seoul 08826, Korea. kaang@snu.ac.kr
- KMID: 2450491
- DOI: http://doi.org/10.4196/kjpp.2019.23.4.237
Abstract
- Confirming the direct link between neural circuit activity and animal behavior has been a principal aim of neuroscience. The genetically encoded calcium indicator (GECI), which binds to calcium ions and emits fluorescence visualizing intracellular calcium concentration, enables detection of in vivo neuronal firing activity. Various GECIs have been developed and can be chosen for diverse purposes. These GECI-based signals can be acquired by several tools including two-photon microscopy and microendoscopy for precise or wide imaging at cellular to synaptic levels. In addition, the images from GECI signals can be analyzed with open source codes including constrained non-negative matrix factorization for endoscopy data (CNMF_E) and miniscope 1-photon-based calcium imaging signal extraction pipeline (MIN1PIPE), and considering parameters of the imaged brain regions (e.g., diameter or shape of soma or the resolution of recorded images), the real-time activity of each cell can be acquired and linked with animal behaviors. As a result, GECI signal analysis can be a powerful tool for revealing the functions of neuronal circuits related to specific behaviors.
Keyword
MeSH Terms
Figure
Reference
-
1. Kawashima T, Okuno H, Bito H. A new era for functional labeling of neurons: activity-dependent promoters have come of age. Front Neural Circuits. 2014; 8:37.
Article2. Minatohara K, Akiyoshi M, Okuno H. Role of immediate-early genes in synaptic plasticity and neuronal ensembles underlying the memory trace. Front Mol Neurosci. 2016; 8:78.
Article3. Cho J, Yu NK, Choi JH, Sim SE, Kang SJ, Kwak C, Lee SW, Kim JI, Choi DI, Kim VN, Kaang BK. Multiple repressive mechanisms in the hippocampus during memory formation. Science. 2015; 350:82–87.
Article4. Xiu J, Zhang Q, Zhou T, Zhou TT, Chen Y, Hu H. Visualizing an emotional valence map in the limbic forebrain by TAI-FISH. Nat Neurosci. 2014; 17:1552–1559.
Article5. Zhang Q, He Q, Wang J, Fu C, Hu H. Use of TAI-FISH to visualize neural ensembles activated by multiple stimuli. Nat Protoc. 2018; 13:118–133.
Article6. Yokose J, Okubo-Suzuki R, Nomoto M, Ohkawa N, Nishizono H, Suzuki A, Matsuo M, Tsujimura S, Takahashi Y, Nagase M, Watabe AM, Sasahara M, Kato F, Inokuchi K. Overlapping memory trace indispensable for linking, but not recalling, individual memories. Science. 2017; 355:398–403.
Article7. Choi JH, Sim SE, Kim JI, Choi DI, Oh J, Ye S, Lee J, Kim T, Ko HG, Lim CS, Kaang BK. Interregional synaptic maps among engram cells underlie memory formation. Science. 2018; 360:430–435.
Article8. Denny CA, Kheirbek MA, Alba EL, Tanaka KF, Brachman RA, Laughman KB, Tomm NK, Turi GF, Losonczy A, Hen R. Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron. 2014; 83:189–201.
Article9. Abdou K, Shehata M, Choko K, Nishizono H, Matsuo M, Muramatsu SI, Inokuchi K. Synapse-specific representation of the identity of overlapping memory engrams. Science. 2018; 360:1227–1231.
Article10. Rashid AJ, Yan C, Mercaldo V, Hsiang HL, Park S, Cole CJ, De Cristofaro A, Yu J, Ramakrishnan C, Lee SY, Deisseroth K, Frankland PW, Josselyn SA. Competition between engrams influences fear memory formation and recall. Science. 2016; 353:383–387.
Article11. Kitamura T, Ogawa SK, Roy DS, Okuyama T, Morrissey MD, Smith LM, Redondo RL, Tonegawa S. Engrams and circuits crucial for systems consolidation of a memory. Science. 2017; 356:73–78.
Article12. Roy DS, Kitamura T, Okuyama T, Ogawa SK, Sun C, Obata Y, Yoshiki A, Tonegawa S. Distinct neural circuits for the formation and retrieval of episodic memories. Cell. 2017; 170:1000–1012.
Article13. Allen WE, DeNardo LA, Chen MZ, Liu CD, Loh KM, Fenno LE, Ramakrishnan C, Deisseroth K, Luo L. Thirst-associated preoptic neurons encode an aversive motivational drive. Science. 2017; 357:1149–1155.
Article14. Kitamura T, Pignatelli M, Suh J, Kohara K, Yoshiki A, Abe K, Tonegawa S. Island cells control temporal association memory. Science. 2014; 343:896–901.
Article15. Ye X, Kapeller-Libermann D, Travaglia A, Inda MC, Alberini CM. Direct dorsal hippocampal-prelimbic cortex connections strengthen fear memories. Nat Neurosci. 2017; 20:52–61.
Article16. Lu J, Tucciarone J, Padilla-Coreano N, He M, Gordon JA, Huang ZJ. Selective inhibitory control of pyramidal neuron ensembles and cortical subnetworks by chandelier cells. Nat Neurosci. 2017; 20:1377–1383.
Article17. Inoue KI, Takada M, Matsumoto M. Neuronal and behavioural modulations by pathway-selective optogenetic stimulation of the primate oculomotor system. Nat Commun. 2015; 6:8378.
Article18. Kim S, Yu NK, Shim KW, Kim JI, Kim H, Han DH, Choi JE, Lee SW, Choi DI, Kim MW, Lee DS, Lee K, Galjart N, Lee YS, Lee JH, Kaang BK. Remote memory and cortical synaptic plasticity require neuronal CCCTC-Binding Factor (CTCF). J Neurosci. 2018; 38:5042–5052.
Article19. Kang SJ, Kim S, Lee J, Kwak C, Lee K, Zhuo M, Kaang BK. Inhibition of anterior cingulate cortex excitatory neuronal activity induces conditioned place preference in a mouse model of chronic inflammatory pain. Korean J Physiol Pharmacol. 2017; 21:487–493.
Article20. El-Boustani S, Ip JPK, Breton-Provencher V, Knott GW, Okuno H, Bito H, Sur M. Locally coordinated synaptic plasticity of visual cortex neurons in vivo. Science. 2018; 360:1349–1354.21. Markowitz JE, Gillis WF, Beron CC, Neufeld SQ, Robertson K, Bhagat ND, Peterson RE, Peterson E, Hyun M, Linderman SW, Sabatini BL, Datta SR. The striatum organizes 3D behavior via moment-to-moment action selection. Cell. 2018; 174:44–58.
Article22. Dimitrov D, He Y, Mutoh H, Baker BJ, Cohen L, Akemann W, Knöpfel T. Engineering and characterization of an enhanced fluorescent protein voltage sensor. PLoS One. 2007; 2:e440.
Article23. Siegel MS, Isacoff EY. A genetically encoded optical probe of membrane voltage. Neuron. 1997; 19:735–741.
Article24. Ataka K, Pieribone VA. A genetically targetable fluorescent probe of channel gating with rapid kinetics. Biophys J. 2002; 82(1 Pt 1):509–516.
Article25. Kang BE, Baker BJ. Pado, a fluorescent protein with proton channel activity can optically monitor membrane potential, intracellular pH, and map gap junctions. Sci Rep. 2016; 6:23865.
Article26. Jin L, Han Z, Platisa J, Wooltorton JR, Cohen LB, Pieribone VA. Single action potentials and subthreshold electrical events imaged in neurons with a fluorescent protein voltage probe. Neuron. 2012; 75:779–785.
Article27. Kannan M, Vasan G, Pieribone VA. Optimizing strategies for developing genetically encoded voltage indicators. Front Cell Neurosci. 2019; 13:53.
Article28. Baker BJ, Mutoh H, Dimitrov D, Akemann W, Perron A, Iwamoto Y, Jin L, Cohen LB, Isacoff EY, Pieribone VA, Hughes T, Knöpfel T. Genetically encoded fluorescent sensors of membrane potential. Brain Cell Biol. 2008; 36:53–67.
Article29. Baker BJ, Lee H, Pieribone VA, Cohen LB, Isacoff EY, Knopfel T, Kosmidis EK. Three fluorescent protein voltage sensors exhibit low plasma membrane expression in mammalian cells. J Neurosci Methods. 2007; 161:32–38.
Article30. Yang HH, St-Pierre F, Sun X, Ding X, Lin MZ, Clandinin TR. Subcellular imaging of voltage and calcium signals reveals neural processing in vivo. Cell. 2016; 166:245–257.31. Platisa J, Vasan G, Yang A, Pieribone VA. Directed evolution of key residues in fluorescent protein inverses the polarity of voltage sensitivity in the genetically encoded indicator ArcLight. ACS Chem Neurosci. 2017; 8:513–523.
Article32. Iamshanova O, Mariot P, Lehen'kyi V, Prevarskaya N. Comparison of fluorescence probes for intracellular sodium imaging in prostate cancer cell lines. Eur Biophys J. 2016; 45:765–777.
Article33. Kaihara A, Sunami A, Kurokawa J, Furukawa T. A genetically encoded bioluminescent indicator for the sodium channel activity in living cells. J Am Chem Soc. 2009; 131:4188–4189.
Article34. Kandel ER, Schwartz JH, Jessell TM. Principles of neural science. 4th ed. New York: McGraw-Hill;2000.35. Zerangue N, Kavanaugh MP. Flux coupling in a neuronal glutamate transporter. Nature. 1996; 383:634–637.
Article36. Grienberger C, Konnerth A. Imaging calcium in neurons. Neuron. 2012; 73:862–885.
Article37. Inoue M, Takeuchi A, Horigane S, Ohkura M, Gengyo-Ando K, Fujii H, Kamijo S, Takemoto-Kimura S, Kano M, Nakai J, Kitamura K, Bito H. Rational design of a high-affinity, fast, red calcium indicator R-CaMP2. Nat Methods. 2015; 12:64–70.
Article38. Kamijo S, Ishii Y, Horigane SI, Suzuki K, Ohkura M, Nakai J, Fujii H, Takemoto-Kimura S, Bito H. A critical neurodevelopmental role for L-type voltage-gated calcium channels in neurite extension and radial migration. J Neurosci. 2018; 38:5551–5566.
Article39. Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003; 4:517–529.
Article40. Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000; 1:11–21.
Article41. Atlas D. The voltage-gated calcium channel functions as the molecular switch of synaptic transmission. Annu Rev Biochem. 2013; 82:607–635.
Article42. Kim HL, Chang YJ, Lee SM, Hong YS. Genomic structure of the regulatory region of the voltage-gated calcium channel alpha 1D. Exp Mol Med. 1998; 30:246–251.43. Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010; 50:295–322.
Article44. Prakriya M, Lewis RS. Store-operated calcium channels. Physiol Rev. 2015; 95:1383–1436.
Article45. Bagur R, Hajnóczky G. Intracellular Ca2+ sensing: its role in calcium homeostasis and signaling. Mol Cell. 2017; 66:780–788.46. Zahradník I, Györke S, Zahradníková A. Calcium activation of ryanodine receptor channels--reconciling RyR gating models with tetrameric channel structure. J Gen Physiol. 2005; 126:515–527.47. Kania E, Roest G, Vervliet T, Parys JB, Bultynck G. IP3 receptormediated calcium signaling and its role in autophagy in cancer. Front Oncol. 2017; 7:140.
Article48. Glancy B, Balaban RS. Role of mitochondrial Ca2+ in the regulation of cellular energetics. Biochemistry. 2012; 51:2959–2973.49. Schwaller B. Cytosolic Ca2+ buffers. Cold Spring Harb Perspect Biol. 2010; 2:a004051.50. Larkum ME, Watanabe S, Nakamura T, Lasser-Ross N, Ross WN. Synaptically activated Ca2+ waves in layer 2/3 and layer 5 rat neocortical pyramidal neurons. J Physiol. 2003; 549(Pt 2):471–488.51. Reddish FN, Miller CL, Gorkhali R, Yang JJ. Calcium dynamics mediated by the endoplasmic/sarcoplasmic reticulum and related diseases. Int J Mol Sci. 2017; 18:E1024.52. Shimomura O, Johnson FH, Saiga Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J Cell Comp Physiol. 1962; 59:223–239.53. Brown JE, Cohen LB, De Weer P, Pinto LH, Ross WN, Salzberg BM. Rapid changes in intracellular free calcium concentration. Detection by metallochromic indicator dyes in squid giant axon. Biophys J. 1975; 15:1155–1116.
Article54. Paredes RM, Etzler JC, Watts LT, Zheng W, Lechleiter JD. Chemical calcium indicators. Methods. 2008; 46:143–151.
Article55. Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997; 388:882–887.56. Tallini YN, Ohkura M, Choi BR, Ji G, Imoto K, Doran R, Lee J, Plan P, Wilson J, Xin HB, Sanbe A, Gulick J, Mathai J, Robbins J, Salama G, Nakai J, Kotlikoff MI. Imaging cellular signals in the heart in vivo: cardiac expression of the high-signal Ca2+ indicator GCaMP2. Proc Natl Acad Sci U S A. 2006; 103:4753–4758.57. Akerboom J, Rivera JD, Guilbe MM, Malavé EC, Hernandez HH, Tian L, Hires SA, Marvin JS, Looger LL, Schreiter ER. Crystal structures of the GCaMP calcium sensor reveal the mechanism of fluorescence signal change and aid rational design. J Biol Chem. 2009; 284:6455–6464.
Article58. Tang S, Wong HC, Wang ZM, Huang Y, Zou J, Zhuo Y, Pennati A, Gadda G, Delbono O, Yang JJ. Design and application of a class of sensors to monitor Ca2+ dynamics in high Ca2+ concentration cellular compartments. Proc Natl Acad Sci U S A. 2011; 108:16265–16270.59. Jares-Erijman EA, Jovin TM. FRET imaging. Nat Biotechnol. 2003; 21:1387–1395.
Article60. Nakamura T, Barbara JG, Nakamura K, Ross WN. Synergistic release of Ca2+ from IP3-sensitive stores evoked by synaptic activation of mGluRs paired with backpropagating action potentials. Neuron. 1999; 24:727–737.61. Pérez Koldenkova V, Nagai T. Genetically encoded Ca2+ indicators: properties and evaluation. Biochim Biophys Acta. 2013; 1833:1787–1797.62. Nakai J, Ohkura M, Imoto K. A high signal-to-noise Ca2+ probe composed of a single green fluorescent protein. Nat Biotechnol. 2001; 19:137–141.63. Reiff DF, Ihring A, Guerrero G, Isacoff EY, Joesch M, Nakai J, Borst A. In vivo performance of genetically encoded indicators of neural activity in flies. J Neurosci. 2005; 25:4766–4778.64. Ohkura M, Matsuzaki M, Kasai H, Imoto K, Nakai J. Genetically encoded bright Ca2+ probe applicable for dynamic Ca2+ imaging of dendritic spines. Anal Chem. 2005; 77:5861–5869.65. Zhao Y, Araki S, Wu J, Teramoto T, Chang YF, Nakano M, Abdelfattah AS, Fujiwara M, Ishihara T, Nagai T, Campbell RE. An expanded palette of genetically encoded Ca2+ indicators. Science. 2011; 333:1888–1891.66. Akerboom J, Chen TW, Wardill TJ, Tian L, Marvin JS, Mutlu S, Calderón NC, Esposti F, Borghuis BG, Sun XR, Gordus A, Orger MB, Portugues R, Engert F, Macklin JJ, Filosa A, Aggarwal A, Kerr RA, Takagi R, Kracun S, et al. Optimization of a GCaMP calcium indicator for neural activity imaging. J Neurosci. 2012; 32:13819–13840.
Article67. Helassa N, Zhang XH, Conte I, Scaringi J, Esposito E, Bradley J, Carter T, Ogden D, Morad M, Török K. Fast-response calmodulin-based fluorescent indicators reveal rapid intracellular calcium dynamics. Sci Rep. 2015; 5:15978.
Article68. Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, Looger LL, Svoboda K, Kim DS. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013; 499:295–300.
Article69. Barnett LM, Hughes TE, Drobizhev M. Deciphering the molecular mechanism responsible for GCaMP6m's Ca2+-dependent change in fluorescence. PLoS One. 2017; 12:e0170934.70. Dana H, Mohar B, Sun Y, Narayan S, Gordus A, Hasseman JP, Tsegaye G, Holt GT, Hu A, Walpita D, Patel R, Macklin JJ, Bargmann CI, Ahrens MB, Schreiter ER, Jayaraman V, Looger LL, Svoboda K, Kim DS. Sensitive red protein calcium indicators for imaging neural activity. Elife. 2016; 5:e12727.
Article71. Nagai T, Sawano A, Park ES, Miyawaki A. Circularly permuted green fluorescent proteins engineered to sense Ca2+. Proc Natl Acad Sci U S A. 2001; 98:3197–3202.72. Baird GS, Zacharias DA, Tsien RY. Circular permutation and receptor insertion within green fluorescent proteins. Proc Natl Acad Sci U S A. 1999; 96:11241–11246.
Article73. Griesbeck O, Baird GS, Campbell RE, Zacharias DA, Tsien RY. Reducing the environmental sensitivity of yellow fluorescent protein. Mechanism and applications. J Biol Chem. 2001; 276:29188–22919.74. Miyawaki A, Griesbeck O, Heim R, Tsien RY. Dynamic and quantitative Ca2+ measurements using improved cameleons. Proc Natl Acad Sci U S A. 1999; 96:2135–2140.75. Nagai T, Yamada S, Tominaga T, Ichikawa M, Miyawaki A. Expanded dynamic range of fluorescent indicators for Ca2+ by circularly permuted yellow fluorescent proteins. Proc Natl Acad Sci U S A. 2004; 101:10554–10559.76. Horikawa K, Yamada Y, Matsuda T, Kobayashi K, Hashimoto M, Matsu-ura T, Miyawaki A, Michikawa T, Mikoshiba K, Nagai T. Spontaneous network activity visualized by ultrasensitive Ca2+ indicators, yellow Cameleon-Nano. Nat Methods. 2010; 7:729–732.77. Palmer AE, Giacomello M, Kortemme T, Hires SA, Lev-Ram V, Baker D, Tsien RY. Ca2+ indicators based on computationally redesigned calmodulin-peptide pairs. Chem Biol. 2006; 13:521–530.78. Heim N, Griesbeck O. Genetically encoded indicators of cellular calcium dynamics based on troponin C and green fluorescent protein. J Biol Chem. 2004; 279:14280–14286.
Article79. Mank M, Reiff DF, Heim N, Friedrich MW, Borst A, Griesbeck O. A FRET-based calcium biosensor with fast signal kinetics and high fluorescence change. Biophys J. 2006; 90:1790–1796.
Article80. Waldeck-Weiermair M, Alam MR, Khan MJ, Deak AT, Vishnu N, Karsten F, Imamura H, Graier WF, Malli R. Spatiotemporal correlations between cytosolic and mitochondrial Ca2+ signals using a novel red-shifted mitochondrial targeted cameleon. PLoS One. 2012; 7:e45917.81. Hires SA, Tian L, Looger LL. Reporting neural activity with genetically encoded calcium indicators. Brain Cell Biol. 2008; 36:69–86.
Article82. Mank M, Griesbeck O. Genetically encoded calcium indicators. Chem Rev. 2008; 108:1550–1564.
Article83. Muto A, Ohkura M, Abe G, Nakai J, Kawakami K. Real-time visualization of neuronal activity during perception. Curr Biol. 2013; 23:307–311.
Article84. Lin MZ, Schnitzer MJ. Genetically encoded indicators of neuronal activity. Nat Neurosci. 2016; 19:1142–1153.
Article85. Helassa N, Podor B, Fine A, Török K. Design and mechanistic insight into ultrafast calcium indicators for monitoring intracellular calcium dynamics. Sci Rep. 2016; 6:38276.
Article86. Akerboom J, Carreras Calderón N, Tian L, Wabnig S, Prigge M, Tolö J, Gordus A, Orger MB, Severi KE, Macklin JJ, Patel R, Pulver SR, Wardill TJ, Fischer E, Schüler C, Chen TW, Sarkisyan KS, Marvin JS, Bargmann CI, Kim DS, et al. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front Mol Neurosci. 2013; 6:2.
Article87. Higuchi-Sanabria R, Garcia EJ, Tomoiaga D, Munteanu EL, Feinstein P, Pon LA. Characterization of fluorescent proteins for three- and four-color live-cell imaging in S. cerevisiae. PLoS One. 2016; 11:e014612.
Article88. Wäldchen S, Lehmann J, Klein T, van de, Sauer M. Light-induced cell damage in live-cell super-resolution microscopy. Sci Rep. 2015; 5:15348.
Article89. Shaner NC, Steinbach PA, Tsien RY. A guide to choosing fluorescent proteins. Nat Methods. 2005; 2:905–909.
Article90. Adamsky A, Kol A, Kreisel T, Doron A, Ozeri-Engelhard N, Melcer T, Refaeli R, Horn H, Regev L, Groysman M, London M, Goshen I. Astrocytic activation generates de novo neuronal potentiation and memory enhancement. Cell. 2018; 174:59–71.e14.
Article91. Pawley JB. Handbook of biological confocal microscopy. 3rd ed. Boston: Springer US;2006.92. Zong W, Wu R, Li M, Hu Y, Li Y, Li J, Rong H, Wu H, Xu Y, Lu Y, Jia H, Fan M, Zhou Z, Zhang Y, Wang A, Chen L, Cheng H. Fast high-resolution miniature two-photon microscopy for brain imaging in freely behaving mice. Nat Methods. 2017; 14:713–719.
Article93. Zhang L, Liang B, Barbera G, Hawes S, Zhang Y, Stump K, Baum I, Yang Y, Li Y, Lin DT. Miniscope GRIN lens system for calcium imaging of neuronal activity from deep brain structures in behaving animals. Curr Protoc Neurosci. 2019; 86:e56.
Article94. Doi A, Oketani R, Nawa Y, Fujita K. High-resolution imaging in two-photon excitation microscopy using in situ estimations of the point spread function. Biomed Opt Express. 2017; 9:202–213.
Article95. Kobat D, Horton NG, Xu C. In vivo two-photon microscopy to 1.6-mm depth in mouse cortex. J Biomed Opt. 2011; 16:10601.96. Ntziachristos V. Going deeper than microscopy: the optical imaging frontier in biology. Nat Methods. 2010; 7:603–614.
Article97. UCLA Miniscope. Overview of system components [Internet]. UCLA Miniscope;cited 2018 Nov 11. Available from: http://miniscope.org/index.php/Overview_of_System_Components.98. Resendez SL, Jennings JH, Ung RL, Namboodiri VM, Zhou ZC, Otis JM, Nomura H, McHenry JA, Kosyk O, Stuber GD. Visualization of cortical, subcortical and deep brain neural circuit dynamics during naturalistic mammalian behavior with head-mounted microscopes and chronically implanted lenses. Nat Protoc. 2016; 11:566–597.
Article99. Yan W, Peng X, Lin D, Wang Q, Gao J, Luo T, Zhou J, Ye T, Qu J, Niu H. Fluorescence microendoscopy imaging based on GRIN lenses with one- and two-photon excitation modes. Front Optoelectron. 2015; 8:177–182.
Article100. Yan W, Peng X, Lin D, Wang Q, Gao J, Zhou J, Ye T, Qu J, Niu H. Two-photon excited fluorescence microendoscopic imaging using a GRIN lens. In : König K, editor. Multiphoton microscopy in the biomedical sciences XV. Bellingham: SPIE;2015.101. Shuman T, Aharoni D, Cai DJ, Lee CR, Chavlis S, Taxidis J, Flores SE, Cheng K, Javaherian M, Kaba CC, Shtrahman M, Bakhurin KI, Masmanidis S, Khakh BS, Poirazi P, Silva AJ, Golshani P. Breakdown of spatial coding and neural synchronization in epilepsy. bioRxiv. 2018; DOI: 10.1101/358580.
Article102. Ghosh KK, Burns LD, Cocker ED, Nimmerjahn A, Ziv Y, Gamal AE, Schnitzer MJ. Miniaturized integration of a fluorescence microscope. Nat Methods. 2011; 8:871–878.
Article103. Evans DA, Stempel AV, Vale R, Ruehle S, Lefler Y, Branco T. A synaptic threshold mechanism for computing escape decisions. Nature. 2018; 558:590–594.
Article104. Helmchen F, Fee MS, Tank DW, Denk W. A miniature head-mounted two-photon microscope. high-resolution brain imaging in freely moving animals. Neuron. 2001; 31:903–991.105. Silva AJ. Miniaturized two-photon microscope: seeing clearer and deeper into the brain. Light Sci Appl. 2017; 6:e17104.
Article106. Koizumi K, Inoue M, Chowdhury S, Bito H, Yamanaka A, Ishizuka T, Yawo H. Functional emergence of a column-like architecture in layer 5 of mouse somatosensory cortex in vivo. J Physiol Sci. 2019; 69:65–77.107. Andermann ML, Gilfoy NB, Goldey GJ, Sachdev RN, Wölfel M, McCormick DA, Reid RC, Levene MJ. Chronic cellular imaging of entire cortical columns in awake mice using microprisms. Neuron. 2013; 80:900–913.
Article108. Chia TH, Levene MJ. Microprisms for in vivo multilayer cortical imaging. J Neurophysiol. 2009; 102:1310–1314.109. Stamatakis AM, Schachter MJ, Gulati S, Zitelli KT, Malanowski S, Tajik A, Fritz C, Trulson M, Otte SL. Simultaneous optogenetics and cellular resolution calcium imaging during active behavior using a miniaturized microscope. Front Neurosci. 2018; 12:496.
Article110. Briggman KL, Kristan WB. Multifunctional pattern-generating circuits. Annu Rev Neurosci. 2008; 31:271–294.
Article111. Romano SA, Pérez-Schuster V, Jouary A, Boulanger-Weill J, Candeo A, Pietri T, Sumbre G. An integrated calcium imaging processing toolbox for the analysis of neuronal population dynamics. PLoS Comput Biol. 2017; 13:e1005526.
Article112. Mukamel EA, Nimmerjahn A, Schnitzer MJ. Automated analysis of cellular signals from large-scale calcium imaging data. Neuron. 2009; 63:747–760.
Article113. Pnevmatikakis EA, Soudry D, Gao Y, Machado TA, Merel J, Pfau D, Reardon T, Mu Y, Lacefield C, Yang W, Ahrens M, Bruno R, Jessell TM, Peterka DS, Yuste R, Paninski L. Simultaneous denoising, deconvolution, and demixing of calcium imaging data. Neuron. 2016; 89:285–299.
Article114. Lu J, Li C, Singh-Alvarado J, Zhou ZC, Fröhlich F, Mooney R, Wang F. MIN1PIPE: a miniscope 1-photon-based calcium imaging signal extraction pipeline. Cell Rep. 2018; 23:3673–3684.
Article115. Zhou P, Resendez SL, Rodriguez-Romaguera J, Jimenez JC, Neufeld SQ, Giovannucci A, Friedrich J, Pnevmatikakis EA, Stuber GD, Hen R, Kheirbek MA, Sabatini BL, Kass RE, Paninski L. Efficient and accurate extraction of in vivo calcium signals from microendoscopic video data. Elife. 2018; 7:e28728.
Article116. Giovannucci A, Friedrich J, Gunn P, Kalfon J, Brown BL, Koay SA, Taxidis J, Najafi F, Gauthier JL, Zhou P, Khakh BS, Tank DW, Chklovskii DB, Pnevmatikakis EA. CaImAn an open source tool for scalable calcium imaging data analysis. Elife. 2019; 8:e38173.
Article117. Tegtmeier J, Brosch M, Janitzky K, Heinze HJ, Ohl FW, Lippert MT. CAVE: an open-source tool for combined analysis of head-mounted calcium imaging and behavior in MATLAB. Front Neurosci. 2018; 12:958.
Article118. Friedrich J, Zhou P, Paninski L. Fast online deconvolution of calcium imaging data. PLoS Comput Biol. 2017; 13:e1005423.
Article119. Mao BQ, Hamzei-Sichani F, Aronov D, Froemke RC, Yuste R. Dynamics of spontaneous activity in neocortical slices. Neuron. 2001; 32:883–898.
Article120. Greenberg DS, Houweling AR, Kerr JN. Population imaging of ongoing neuronal activity in the visual cortex of awake rats. Nat Neurosci. 2008; 11:749–751.
Article121. Holekamp TF, Turaga D, Holy TE. Fast three-dimensional fluorescence imaging of activity in neural populations by objective-coupled planar illumination microscopy. Neuron. 2008; 57:661–672.
Article122. Sasaki T, Takahashi N, Matsuki N, Ikegaya Y. Fast and accurate detection of action potentials from somatic calcium fluctuations. J Neurophysiol. 2008; 100:1668–1676.
Article123. Balkenius A, Johansson AJ, Balkenius C. Comparing analysis methods in functional calcium imaging of the insect brain. PLoS One. 2015; 10:e0129614.
Article124. Cai DJ, Aharoni D, Shuman T, Shobe J, Biane J, Song W, Wei B, Veshkini M, La-Vu M, Lou J, Flores SE, Kim I, Sano Y, Zhou M, Baumgaertel K, Lavi A, Kamata M, Tuszynski M, Mayford M, Golshani P, et al. A shared neural ensemble links distinct contextual memories encoded close in time. Nature. 2016; 534:115–118.
Article125. Sheintuch L, Rubin A, Brande-Eilat N, Geva N, Sadeh N, Pinchasof O, Ziv Y. Tracking the same neurons across multiple days in Ca2+ imaging data. Cell Rep. 2017; 21:1102–1115.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In-vivo Optical Measurement of Neural Activity in the Brain
- Simultaneous Cellular Imaging, Electrical Recording and Stimulation of Hippocampal Activity in Freely Behaving Mice
- Neural circuit remodeling and structural plasticity in the cortex during chronic pain
- How Inflammation Affects the Brain in Depression: A Review of Functional and Structural MRI Studies
- A Review on Brain Imaging Studies of Suicide in Youth