Korean J Physiol Pharmacol.
2019 Jul;23(4):231-236. 10.4196/kjpp.2019.23.4.231.
Potency and plasma protein binding of drugs in vitro—a potentially misleading pair for predicting in vivo efficacious concentrations in humans
- Affiliations
-
- 1Department of Clinical Pharmacology and Therapeutics, The Catholic University of Korea, Seoul St. Mary's Hospital, Seoul 06591, Korea. yimds@catholic.ac.kr
- 2PIPET (Pharmacometrics Institute for Practical Education and Training), College of Medicine, The Catholic University of Korea, Seoul 06591, Korea.
- KMID: 2450490
- DOI: http://doi.org/10.4196/kjpp.2019.23.4.231
Abstract
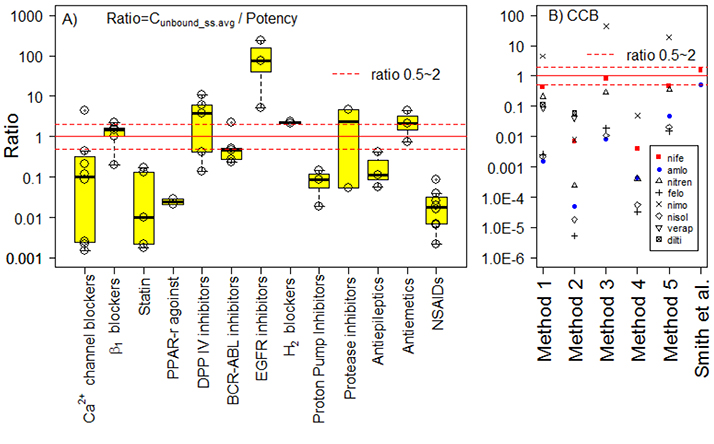
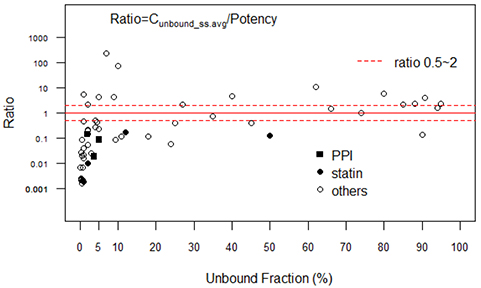
- In drug discovery or preclinical stages of development, potency parameters such as ICâ‚…â‚€, K(i), or K(d) in vitro have been routinely used to predict the parameters of efficacious exposure (AUC, C(min), etc.) in humans. However, to our knowledge, the fundamental assumption that the potency in vitro is correlated with the efficacious concentration in vivo in humans has not been investigated extensively. Thus, the present review examined this assumption by comparing a wide range of published pharmacokinetic (PK) and potency data. If the drug potency in vitro and its in vivo effectiveness in humans are well correlated, the steady-state average unbound concentrations in humans [C(u_ss.avg) = f(u)·F·Dose/(CL·Ï„) = f(u)·AUCss/Ï„] after treatment with approved dosage regimens should be higher than, or at least comparable to, the potency parameters assessed in vitro. We reviewed the ratios of C(u_ss.avg)/potency in vitro for a total of 54 drug entities (13 major therapeutic classes) using the dosage, PK, and in vitro potency reported in the published literature. For 54 drugs, the C(u_ss.avg)/in vitro potency ratios were < 1 for 38 (69%) and < 0.1 for 22 (34%) drugs. When the ratios were plotted against f(u) (unbound fraction), "ratio < 1" was predominant for drugs with high protein binding (90% of drugs with f(u) ≤ 5%; i.e., 28 of 31 drugs). Thus, predicting the in vivo efficacious unbound concentrations in humans using only in vitro potency data and f(u) should be avoided, especially for molecules with high protein binding.
Keyword
Figure
Reference
-
1. Smith DA, Di L, Kerns EH. The effect of plasma protein binding on in vivo efficacy: misconceptions in drug discovery. Nat Rev Drug Discov. 2010; 9:929–939.
Article2. Obach RS, Lombardo F, Waters NJ. Trend analysis of a database of intravenous pharmacokinetic parameters in humans for 670 drug compounds. Drug Metab Dispos. 2008; 36:1385–1405.
Article3. Chiorean EG, Porter JM, Foster AE, Al Omari AS, Yoder CA, Fife KL, Strother RM, Murry DJ, Yu M, Jones DR, Sweeney CJ. A phase I and pharmacokinetic trial of erlotinib in combination with weekly docetaxel in patients with taxane-naïve malignancies. Clin Cancer Res. 2008; 14:1131–1137.4. Liao J, Gallas M, Pegram M, Slingerland J. Lapatinib: new opportunities for management of breast cancer. Breast Cancer. 2010; 2:79–91.5. Eadie MJ. Therapeutic drug monitoring--antiepileptic drugs. Br J Clin Pharmacol. 1998; 46:185–193.6. Sun J, Triggle DJ. Calcium channel antagonists: cardiovascular selectivity of action. J Pharmacol Exp Ther. 1995; 274:419–426.7. Baker JG. The selectivity of beta-adrenoceptor antagonists at the human beta1, beta2 and beta3 adrenoceptors. Br J Pharmacol. 2005; 144:317–322.8. Buckett L, Ballard P, Davidson R, Dunkley C, Martin L, Stafford J, McTaggart F. Selectivity of ZD4522 for inhibition of cholesterol synthesis in hepatic versus non-hepatic cells. Atherosclerosis. 2000; 151:41.
Article9. Xu HE, Lambert MH, Montana VG, Plunket KD, Moore LB, Collins JL, Oplinger JA, Kliewer SA, Gampe RT Jr, McKee DD, Moore JT, Willson TM. Structural determinants of ligand binding selectivity between the peroxisome proliferator-activated receptors. Proc Natl Acad Sci U S A. 2001; 98:13919–13924.
Article10. Thomas L, Eckhardt M, Langkopf E, Tadayyon M, Himmelsbach F, Mark M. (R)-8-(3-amino-piperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylm ethyl)-3,7-dihydro-purine-2,6-dione (BI 1356), a novel xanthine-based dipeptidyl peptidase 4 inhibitor, has a superior potency and longer duration of action compared with other dipeptidyl peptidase-4 inhibitors. J Pharmacol Exp Ther. 2008; 325:175–182.11. Redaelli S, Mologni L, Rostagno R, Piazza R, Magistroni V, Ceccon M, Viltadi M, Flynn D, Gambacorti-Passerini C. Three novel patient-derived BCR/ABL mutants show different sensitivity to second and third generation tyrosine kinase inhibitors. Am J Hematol. 2012; 87:E125–E128.
Article12. Kitagawa D, Yokota K, Gouda M, Narumi Y, Ohmoto H, Nishiwaki E, Akita K, Kirii Y. Activity-based kinase profiling of approved tyrosine kinase inhibitors. Genes Cells. 2013; 18:110–122.
Article13. Gustavsson S, Mårdh S, Norberg L, Nyrén O, Wollert S. Omeprazole, cimetidine, and ranitidine: inhibition of acid production in isolated human parietal cells. Scand J Gastroenterol. 1985; 20:917–921.
Article14. Bastaki SM, Chandranath I, Garner A. Comparison of five antisecretory agents acting via gastric H+/K+-ATPase. J Physiol Paris. 2000; 94:19–23.15. Stevens R, Kakuda T, Bertz R, Mo H, Molla A, Rode R, Kempf D. Inhibitory quotient of protease inhibitors using a standardized determination of IC50. In : 4th International Workshop on Clinical Pharmacology of HIV Therapy; 2003 Mar 27–29; Cannes, France.16. Lang DG, Wang CM, Cooper BR. Lamotrigine, phenytoin and carbamazepine interactions on the sodium current present in N4TG1 mouse neuroblastoma cells. J Pharmacol Exp Ther. 1993; 266:829–835.17. Ito H, Hidaka K, Miyata K, Kamato T, Nishida A, Honda K. Characterization of YM060, a potent and selective 5-hydroxytryptamine3 receptor antagonist, in rabbit nodose ganglion and N1E-115 neuroblastoma cells. J Pharmacol Exp Ther. 1992; 263:1127–1132.18. Warner TD, Giuliano F, Vojnovic I, Bukasa A, Mitchell JA, Vane JR. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc Natl Acad Sci U S A. 1999; 96:7563–7568.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Does 3-O-Methyldopa(3-OMD) Inhibit the Binding of Levodopa to Plasma Protein
- Antidepressants and Related Drug Interactions
- Drug-drug Interactions between Psychotropic Agents and Other Drugs in Physically Ill Patients: Experience of Consultation-liason in Korea University Hospital
- Clinical Significances of Scrum Protein C and S in Chronic Renal Failure
- Studies on the Specific Estradiol Binding Site on Rat Uterine Membranes