Yonsei Med J.
2015 May;56(3):798-804. 10.3349/ymj.2015.56.3.798.
The Difference of Lymphocyte Subsets Including Regulatory T-Cells in Umbilical Cord Blood between AGA Neonates and SGA Neonates
- Affiliations
-
- 1Division of Maternal and Fetal Medicine, Department of Obstetrics and Gynecology, Research Institute of Medical Science, Konkuk University School of Medicine, Seoul, Korea. 19960011@kuh.ac.kr
- 2Department of Laboratory Medicine, Konkuk University School of Medicine, Seoul, Korea.
- KMID: 2450356
- DOI: http://doi.org/10.3349/ymj.2015.56.3.798
Abstract
- PURPOSE
This study aimed to compare the regulatory T cells in cord blood of appropriate for gestational age (AGA) neonates with those of small for gestational age (SGA) neonates.
MATERIALS AND METHODS
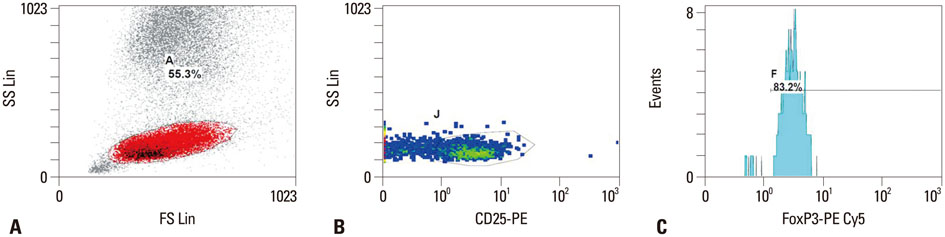
Umbilical cord blood was collected upon labor in 108 healthy full-term (between 37 and 41 gestational weeks) neonates, who were born between November 2010 and April 2012. Among them, 77 samples were obtained from AGA neonates, and 31 samples were obtained from SGA neonates. Regulatory T cells and lymphocyte subsets were determined using a flow cytometer. Student's t-test for independent samples was used to compare differences between AGA and SGA neonates.
RESULTS
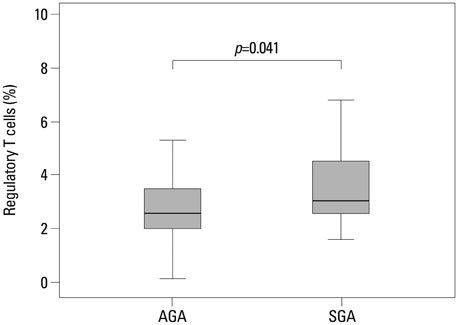
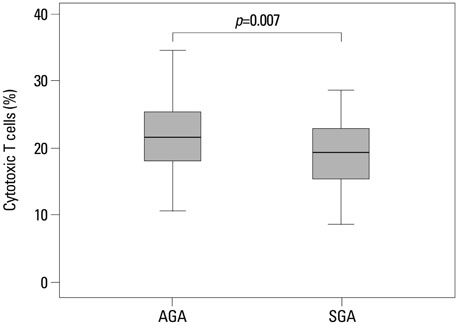
Regulatory T cells in cord blood were increased in the SGA group compared with normal controls (p=0.041). However, cytotoxic T cells in cord blood were significantly decreased in the SGA group compared with normal controls (p=0.007).
CONCLUSION
This is the first study to compare the distribution of lymphocyte subsets including regulatory T cells in cord blood between AGA neonates and SGA neonates.
MeSH Terms
Figure
Reference
-
1. Cunningham FG, Leveno KJ, Bloom SL, Hauth JC, Gilstrap LC, Wenstrom KD. Williams Obstetrics. 22nd ed. New York: McGraw-Hill;2005. p. 51–82.2. Chan PY, Morris JM, Leslie GI, Kelly PJ, Gallery ED. The long-term effects of prematurity and intrauterine growth restriction on cardiovascular, renal, and metabolic function. Int J Pediatr. 2010; 2010:280402.
Article3. Raqib R, Alam DS, Sarker P, Ahmad SM, Ara G, Yunus M, et al. Low birth weight is associated with altered immune function in rural Bangladeshi children: a birth cohort study. Am J Clin Nutr. 2007; 85:845–852.
Article4. Go SW, Kim KJ, Kim MY, Kim SJ, Kim SY, Kim SJ, et al. Obstetrics. Korean society of obstetrics and gynecology. 4th ed. Seoul: Koonja company;2007. p. 628–631.5. Al Qahtani N. Doppler ultrasound in the assessment of suspected intra-uterine growth restriction. Ann Afr Med. 2011; 10:266–271.
Article6. Amarilyo G, Oren A, Mimouni FB, Ochshorn Y, Deutsch V, Mandel D. Increased cord serum inflammatory markers in small-for-gestational-age neonates. J Perinatol. 2011; 31:30–32.
Article7. Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011; 146:772–784.
Article8. Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+CD25 high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007; 178:2579–2588.
Article9. Ben-Shoshan J, Maysel-Auslender S, Mor A, Keren G, George J. Hypoxia controls CD4+CD25+ regulatory T-cell homeostasis via hypoxia-inducible factor-1alpha. Eur J Immunol. 2008; 38:2412–2418.
Article10. Praksova P, Stourac P, Bednarik J, Vlckova E, Mikulkova Z, Michalek J. Immunoregulatory T cells in multiple sclerosis and the effect of interferon beta and glatiramer acetate treatment on T cell subpopulations. J Neurol Sci. 2012; 319:18–23.
Article11. Takahata Y, Nomura A, Takada H, Ohga S, Furuno K, Hikino S, et al. CD25+CD4+ T cells in human cord blood: an immunoregulatory subset with naive phenotype and specific expression of forkhead box p3 (Foxp3) gene. Exp Hematol. 2004; 32:622–629.
Article12. Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000; 101:455–458.13. Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002; 2:389–400.
Article14. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995; 155:1151–1164.15. Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J Immunol. 2002; 168:1080–1086.
Article16. Graca L, Cobbold SP, Waldmann H. Identification of regulatory T cells in tolerated allografts. J Exp Med. 2002; 195:1641–1646.
Article17. Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003; 3:199–210.
Article18. Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004; 112:38–43.
Article19. Mjösberg J, Svensson J, Johansson E, Hellström L, Casas R, Jenmalm MC, et al. Systemic reduction of functionally suppressive CD4dimCD25highFoxp3+ Tregs in human second trimester pregnancy is induced by progesterone and 17beta-estradiol. J Immunol. 2009; 183:759–769.
Article20. Mjösberg J, Berg G, Ernerudh J, Ekerfelt C. CD4+ CD25+ regulatory T cells in human pregnancy: development of a Treg-MLC-ELISPOT suppression assay and indications of paternal specific Tregs. Immunology. 2007; 120:456–466.
Article21. Tilburgs T, Roelen DL, van der Mast BJ, de Groot-Swings GM, Kleijburg C, Scherjon SA, et al. Evidence for a selective migration of fetus-specific CD4+CD25bright regulatory T cells from the peripheral blood to the decidua in human pregnancy. J Immunol. 2008; 180:5737–5745.
Article22. Kim H, Moon HW, Hur M, Park CM, Yun YM, Hwang HS, et al. Distribution of CD4+ CD25 high FoxP3+ regulatory T-cells in umbilical cord blood. J Matern Fetal Neonatal Med. 2012; 25:2058–2061.
Article23. Leber A, Teles A, Zenclussen AC. Regulatory T cells and their role in pregnancy. Am J Reprod Immunol. 2010; 63:445–459.
Article24. Pijnenborg R, Bland JM, Robertson WB, Brosens I. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta. 1983; 4:397–413.
Article25. Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod. 2004; 10:347–353.
Article26. Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004; 5:266–271.
Article27. King A, Loke YW. On the nature and function of human uterine granular lymphocytes. Immunol Today. 1991; 12:432–435.
Article28. Stallmach T, Hebisch G, Joller-Jemelka HI, Orban P, Schwaller J, Engelmann M. Cytokine production and visualized effects in the feto-maternal unit. Quantitative and topographic data on cytokines during intrauterine disease. Lab Invest. 1995; 73:384–392.29. Jasper MJ, Tremellen KP, Robertson SA. Primary unexplained infertility is associated with reduced expression of the T-regulatory cell transcription factor Foxp3 in endometrial tissue. Mol Hum Reprod. 2006; 12:301–308.
Article30. Mackroth MS, Malhotra I, Mungai P, Koech D, Muchiri E, King CL. Human cord blood CD4+CD25hi regulatory T cells suppress prenatally acquired T cell responses to Plasmodium falciparum antigens. J Immunol. 2011; 186:2780–2791.
Article31. Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010; 10:490–500.
Article32. Wang GL, Semenza GL. Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J Biol Chem. 1993; 268:21513–21518.
Article33. Pringle KG, Kind KL, Sferruzzi-Perri AN, Thompson JG, Roberts CT. Beyond oxygen: complex regulation and activity of hypoxia inducible factors in pregnancy. Hum Reprod Update. 2010; 16:415–431.
Article34. Rajakumar A, Jeyabalan A, Markovic N, Ness R, Gilmour C, Conrad KP. Placental HIF-1 alpha, HIF-2 alpha, membrane and soluble VEGF receptor-1 proteins are not increased in normotensive pregnancies complicated by late-onset intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol. 2007; 293:R766–R774.35. Conforti L, Petrovic M, Mohammad D, Lee S, Ma Q, Barone S, et al. Hypoxia regulates expression and activity of Kv1.3 channels in T lymphocytes: a possible role in T cell proliferation. J Immunol. 2003; 170:695–702.
Article36. Robbins JR, Lee SM, Filipovich AH, Szigligeti P, Neumeier L, Petrovic M, et al. Hypoxia modulates early events in T cell receptor-mediated activation in human T lymphocytes via Kv1.3 channels. J Physiol. 2005; 564(Pt 1):131–143.
Article37. Sitkovsky M, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat Rev Immunol. 2005; 5:712–721.
Article38. Romagnani S. Type 1 T helper and type 2 T helper cells: functions, regulation and role in protection and disease. Int J Clin Lab Res. 1991; 21:152–158.
Article39. Del Prete G. Human Th1 and Th2 lymphocytes: their role in the pathophysiology of atopy. Allergy. 1992; 47:450–455.
Article40. Weetman AP. The immunology of pregnancy. Thyroid. 1999; 9:643–646.
Article41. Engel SA, Olshan AF, Savitz DA, Thorp J, Erichsen HC, Chanock SJ. Risk of small-for-gestational age is associated with common anti-inflammatory cytokine polymorphisms. Epidemiology. 2005; 16:478–486.
Article42. Dealtry GB, O'Farrell MK, Fernandez N. The Th2 cytokine environment of the placenta. Int Arch Allergy Immunol. 2000; 123:107–119.
Article43. Raghupathy R, Makhseed M, Azizieh F, Hassan N, Al-Azemi M, Al-Shamali E. Maternal Th1- and Th2-type reactivity to placental antigens in normal human pregnancy and unexplained recurrent spontaneous abortions. Cell Immunol. 1999; 196:122–130.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of umbilical venous erythropoietin concentration between appropriate and small-for-gestational-age neonates of term pregnancy
- Study of umbilical cord blood adiponectin, IGF-I, IGFBP-1, insulin and leptin levels in small for gestational age neonates at birth
- The significance of nucleated red blood cell counts in low birth weight neonates
- Comparison of levels of umbilical venous erythropoietin and nucleated erythrocytes between appropriate and small for gestational age preterm neonates
- Cord Blood Adiponectin and Insulin-like Growth Factor-I in Term Neonates of Gestational Diabetes Mellitus Mothers: Relationship to Fetal Growth