Yonsei Med J.
2015 May;56(3):778-784. 10.3349/ymj.2015.56.3.778.
Effect of Antifreeze Protein on Mouse Ovarian Tissue Cryopreservation and Transplantation
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Seoul National University Bundang Hospital, Seongnam, Korea.
- 2Department of Obstetrics and Gynecology, Seoul National University College of Medicine, Seoul, Korea. seokhyun@snu.ac.kr
- KMID: 2450353
- DOI: http://doi.org/10.3349/ymj.2015.56.3.778
Abstract
- PURPOSE
To investigate the effect of antifreeze protein (AFP) supplementation on ovarian vitrification and transplantation.
MATERIALS AND METHODS
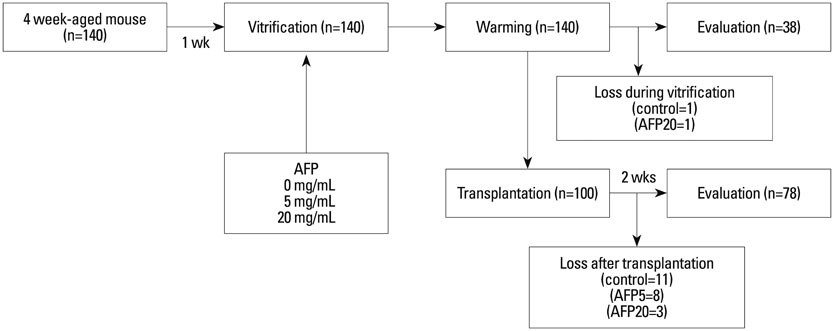
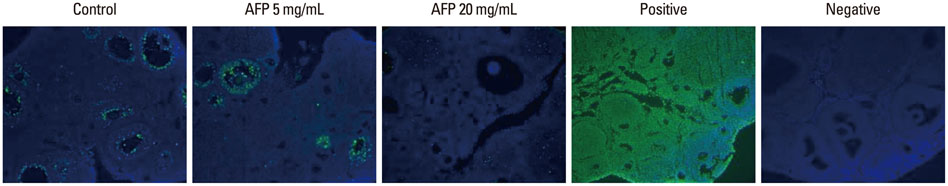
In this experimental study, we researched a total of 182 ovaries from 4-week-old ICR mice. The equilibration solution included 20% ethylene glycol (EG), and the vitrification solution included 40% EG, 18% Ficoll, and 0.3 M sucrose. Intact ovaries were first suspended in 1 mL of equilibration solution for 10 min, and then mixed with 0.5 mL of vitrification solution for 5 min. Ovaries were randomly assigned to 3 groups and 0, 5, or 20 mg/mL of type III AFP was added into the vitrification solution (control, AFP5, and AFP20 groups, respectively). The vitrified ovaries were evaluated after warming and 2 weeks after autotransplantation. The main outcome measurements are follicular morphology and apoptosis assessed by histology and the TUNEL assay.
RESULTS
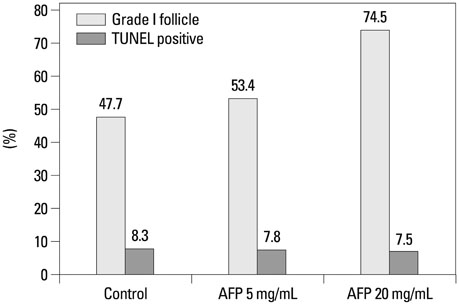
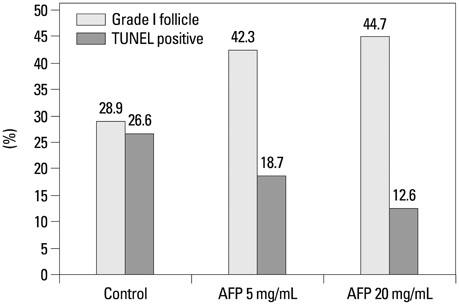
A significantly higher intact follicle ratio was shown in the AFP treated groups (control, 28.9%; AFP5, 42.3%; and AFP20, 44.7%). The rate of apoptotic follicles was significantly lower in the AFP treated groups (control, 26.6%; AFP5, 18.7%; and AFP20, 12.6%). After transplantation of the vitrified-warmed ovaries, a significantly higher intact follicle ratio was shown in the AFP20 group. The rate of apoptotic follicles was similar among the groups.
CONCLUSION
The results of the present study suggest that supplementing AFP in the vitrification solution has beneficial effects on the survival of ovarian tissue during cryopreservation and transplantation.
MeSH Terms
Figure
Cited by 1 articles
-
Improvement in Ovarian Tissue Quality with Supplementation of Antifreeze Protein during Warming of Vitrified Mouse Ovarian Tissue
Hyun Sun Kong, Eun Jung Kim, Hye Won Youm, Seul Ki Kim, Jung Ryeol Lee, Chang Suk Suh, Seok Hyun Kim
Yonsei Med J. 2018;59(2):331-336. doi: 10.3349/ymj.2018.59.2.331.
Reference
-
1. Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Orthotopic and heterotopic ovarian tissue transplantation. Hum Reprod Update. 2009; 15:649–665.
Article2. Donnez J, Jadoul P, Pirard C, Hutchings G, Demylle D, Squifflet J, et al. Live birth after transplantation of frozen-thawed ovarian tissue after bilateral oophorectomy for benign disease. Fertil Steril. 2012; 98:720–725.
Article3. Weissman A, Gotlieb L, Colgan T, Jurisicova A, Greenblatt EM, Casper RF. Preliminary experience with subcutaneous human ovarian cortex transplantation in the NOD-SCID mouse. Biol Reprod. 1999; 60:1462–1467.
Article4. Sağsöz N, Kisa U, Apan A. Ischaemia-reperfusion injury of rat ovary and the effects of vitamin C, mannitol and verapamil. Hum Reprod. 2002; 17:2972–2976.
Article5. Sapmaz E, Ayar A, Celik H, Sapmaz T, Kilic N, Yasar MA. Effects of melatonin and oxytetracycline in autologous intraperitoneal ovary transplantation in rats. Neuro Endocrinol Lett. 2003; 24:350–354.6. Karaca M, Odabasoglu F, Kumtepe Y, Albayrak A, Cadirci E, Keles ON. Protective effects of erythropoietin on ischemia/reperfusion injury of rat ovary. Eur J Obstet Gynecol Reprod Biol. 2009; 144:157–162.
Article7. Onions VJ, Mitchell MR, Campbell BK, Webb R. Ovarian tissue viability following whole ovine ovary cryopreservation: assessing the effects of sphingosine-1-phosphate inclusion. Hum Reprod. 2008; 23:606–618.
Article8. Jee BC, Lee JR, Youm H, Suh CS, Kim SH, Moon SY. Effect of sphingosine-1-phosphate supplementation on follicular integrity of vitrified-warmed mouse ovarian grafts. Eur J Obstet Gynecol Reprod Biol. 2010; 152:176–180.
Article9. Schnorr J, Oehninger S, Toner J, Hsiu J, Lanzendorf S, Williams R, et al. Functional studies of subcutaneous ovarian transplants in non-human primates: steroidogenesis, endometrial development, ovulation, menstrual patterns and gamete morphology. Hum Reprod. 2002; 17:612–619.
Article10. Suzuki H, Ishijima T, Maruyama S, Yanagimoto Ueta Y, Abe Y, Saitoh H. Beneficial effect of desialylated erythropoietin administration on the frozen-thawed canine ovarian xenotransplantation. J Assist Reprod Genet. 2008; 25:571–575.
Article11. Yeh Y, Feeney RE. Antifreeze Proteins: Structures and Mechanisms of Function. Chem Rev. 1996; 96:601–618.
Article12. Wang JH. A comprehensive evaluation of the effects and mechanisms of antifreeze proteins during low-temperature preservation. Cryobiology. 2000; 41:1–9.
Article13. Jo JW, Jee BC, Lee JR, Suh CS. Effect of antifreeze protein supplementation in vitrification medium on mouse oocyte developmental competence. Fertil Steril. 2011; 96:1239–1245.
Article14. Prathalingam NS, Holt WV, Revell SG, Mirczuk S, Fleck RA, Watson PF. Impact of antifreeze proteins and antifreeze glycoproteins on bovine sperm during freeze-thaw. Theriogenology. 2006; 66:1894–1900.
Article15. Amir G, Rubinsky B, Horowitz L, Miller L, Leor J, Kassif Y, et al. Prolonged 24-hour subzero preservation of heterotopically transplanted rat hearts using antifreeze proteins derived from arctic fish. Ann Thorac Surg. 2004; 77:1648–1655.
Article16. Martínez-Páramo S, Barbosa V, Pérez-Cerezales S, Robles V, Herráez MP. Cryoprotective effects of antifreeze proteins delivered into zebrafish embryos. Cryobiology. 2009; 58:128–133.
Article17. Chang HJ, Moon JH, Lee JR, Jee BC, Suh CS, Kim SH. Optimal condition of vitrification method for cryopreservation of human ovarian cortical tissues. J Obstet Gynaecol Res. 2011; 37:1092–1101.
Article18. Arav A, Rubinsky B, Fletcher G, Seren E. Cryogenic protection of oocytes with antifreeze proteins. Mol Reprod Dev. 1993; 36:488–493.
Article19. Lundy T, Smith P, O'Connell A, Hudson NL, McNatty KP. Populations of granulosa cells in small follicles of the sheep ovary. J Reprod Fertil. 1999; 115:251–262.
Article20. Gandolfi F, Paffoni A, Papasso Brambilla E, Bonetti S, Brevini TA, Ragni G. Efficiency of equilibrium cooling and vitrification procedures for the cryopreservation of ovarian tissue: comparative analysis between human and animal models. Fertil Steril. 2006; 85:Suppl 1. 1150–1156.
Article21. Davies PL, Hew CL. Biochemistry of fish antifreeze proteins. FASEB J. 1990; 4:2460–2468.
Article22. Raymond JA, DeVries AL. Adsorption inhibition as a mechanism of freezing resistance in polar fishes. Proc Natl Acad Sci U S A. 1977; 74:2589–2593.
Article23. Yang DS, Sax M, Chakrabartty A, Hew CL. Crystal structure of an antifreeze polypeptide and its mechanistic implications. Nature. 1988; 333:232–237.
Article24. Carpenter JF, Hansen TN. Antifreeze protein modulates cell survival during cryopreservation: mediation through influence on ice crystal growth. Proc Natl Acad Sci U S A. 1992; 89:8953–8957.
Article25. Chao H, Davies PL, Carpenter JF. Effects of antifreeze proteins on red blood cell survival during cryopreservation. J Exp Biol. 1996; 199(Pt 9):2071–2076.
Article26. Rubinsky B, Arav A, Devries AL. The cryoprotective effect of antifreeze glycopeptides from antarctic fishes. Cryobiology. 1992; 29:69–79.
Article27. O'Neil L, Paynter SJ, Fuller BJ, Shaw RW, DeVries AL. Vitrification of mature mouse oocytes in a 6 M Me2SO solution supplemented with antifreeze glycoproteins: the effect of temperature. Cryobiology. 1998; 37:59–66.28. Payne SR, Oliver JE, Upreti GC. Effect of antifreeze proteins on the motility of ram spermatozoa. Cryobiology. 1994; 31:180–184.
Article29. Younis AI, Rooks B, Khan S, Gould KG. The effects of antifreeze peptide III (AFP) and insulin transferrin selenium (ITS) on cryopreservation of chimpanzee (Pan troglodytes) spermatozoa. J Androl. 1998; 19:207–214.30. Jo JW, Jee BC, Suh CS, Kim SH. The beneficial effects of antifreeze proteins in the vitrification of immature mouse oocytes. PLoS One. 2012; 7:e37043.
Article31. Bagis H, Akkoç T, TasXMLLink_XYZ A, Aktoprakligil D. Cryogenic effect of antifreeze protein on transgenic mouse ovaries and the production of live offspring by orthotopic transplantation of cryopreserved mouse ovaries. Mol Reprod Dev. 2008; 75:608–613.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ovarian tissue cryopreservation and transplantation
- Improvement in Ovarian Tissue Quality with Supplementation of Antifreeze Protein during Warming of Vitrified Mouse Ovarian Tissue
- Effect of Cryopreservation on the Heat Shock Protein 90 Expression in Mouse Ovarian Tissue
- Ovarian tissue cryopreservation and transplantation for fertility preservation
- Ovarian tissue cryopreservation and transplantation in patients with cancer