Yonsei Med J.
2018 Mar;59(2):331-336. 10.3349/ymj.2018.59.2.331.
Improvement in Ovarian Tissue Quality with Supplementation of Antifreeze Protein during Warming of Vitrified Mouse Ovarian Tissue
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Seoul National University Bundang Hospital, Seongnam, Korea. leejrmd@snu.ac.kr
- 2Department of Obstetrics and Gynecology, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2418799
- DOI: http://doi.org/10.3349/ymj.2018.59.2.331
Abstract
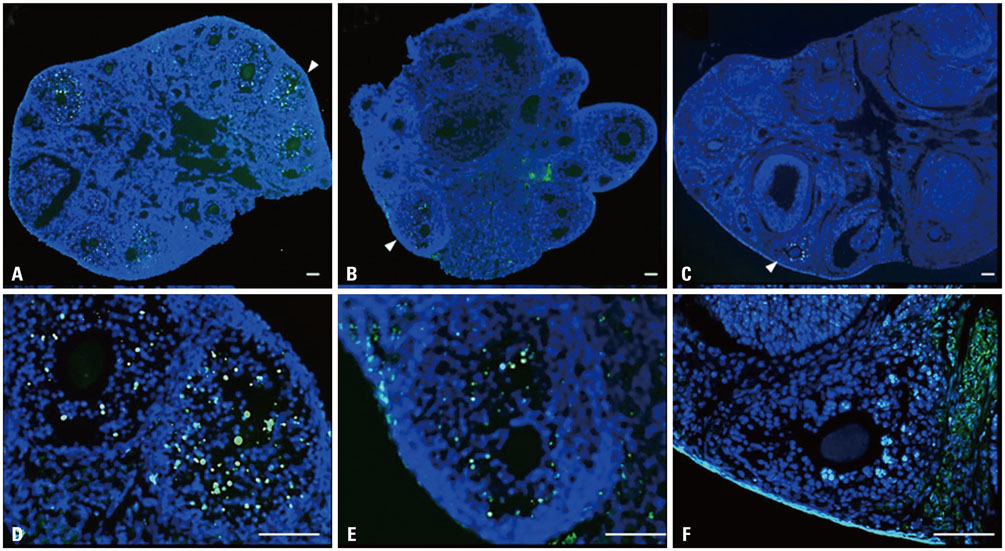
- Ice easily recrystallizes during warming after vitrification, and antifreeze protein (AFP) can inhibit the re-crystallization. However, no study has evaluated the effect of AFP treatment only thereon during warming. This study sought to compare AFP treatment protocols: a conventional protocol with AFP treatment during vitrification and first-step warming and a new protocol with AFP treatment during the first-step warming only. According to the protocols, 10 mg/mL of LeIBP (a type of AFP) was used. Five-week-old B6D2F1 mouse ovaries were randomly divided into a vitrified-warmed control and two experimental groups, one treated with the conventional AFP treatment protocol (LeIBP-all) and the other with the new AFP treatment protocol (LeIBP-w). For evaluation, ratios of ovarian follicle integrity, apoptosis, and DNA double-strand (DDS) damage/repairing were analyzed. The LeIBP-treated groups showed significantly higher intact follicle ratios than the control, and the results were similar between the LeIBP-treated groups. Apoptotic follicle ratios were significantly lower in both LeIBP-treated groups than the control, and the results were not significantly different between the LeIBP-treated groups. With regard to DDS damage/repairing follicle ratio, significantly lower ratios were recorded in both LeIBP-treated groups, compared to the control, and the results were similar between the LeIBP-treated groups. This study demonstrated that both protocols with LeIBP had a beneficial effect on maintaining follicle integrity and preventing follicle apoptosis and DDS damage. Moreover, the new protocol showed similar results to the conventional protocol. This new protocol could optimize the mouse ovary vitrification-warming procedure using AFP, while minimizing the treatment steps.
Keyword
MeSH Terms
Figure
Reference
-
1. DeVries AL. Glycoproteins as biological antifreeze agents in antarctic fishes. Science. 1971; 172:1152–1155.
Article2. Bar Dolev M, Braslavsky I, Davies PL. Ice-binding proteins and their function. Annu Rev Biochem. 2016; 85:515–542.
Article3. Lee J, Kim SK, Youm HW, Kim HJ, Lee JR, Suh CS, et al. Effects of three different types of antifreeze proteins on mouse ovarian tissue cryopreservation and transplantation. PLoS One. 2015; 10:e0126252.
Article4. Lee JR, Youm HW, Lee HJ, Jee BC, Suh CS, Kim SH. Effect of antifreeze protein on mouse ovarian tissue cryopreservation and transplantation. Yonsei Med J. 2015; 56:778–784.
Article5. Morewood T, Getreu N, Fuller B, Morris J, Hardiman P. The effect of thawing protocols on follicle conservation in human ovarian tissue cryopreservation. Cryo Letters. 2017; 38:137–144.6. Seki S, Mazur P. Ultra-rapid warming yields high survival of mouse oocytes cooled to -196°C in dilutions of a standard vitrification solution. PLoS One. 2012; 7:e36058.
Article7. Youm HW, Lee JR, Lee J, Jee BC, Suh CS, Kim SH. Optimal vitrification protocol for mouse ovarian tissue cryopreservation: effect of cryoprotective agents and in vitro culture on vitrified-warmed ovarian tissue survival. Hum Reprod. 2014; 29:720–730.
Article8. Lundy T, Smith P, O'Connell A, Hudson NL, McNatty KP. Populations of granulosa cells in small follicles of the sheep ovary. J Reprod Fertil. 1999; 115:251–262.
Article9. Borges EN, Silva RC, Futino DO, Rocha-Junior CM, Amorim CA, Báo SN, et al. Cryopreservation of swine ovarian tissue: effect of different cryoprotectants on the structural preservation of preantral follicle oocytes. Cryobiology. 2009; 59:195–200.
Article10. Kong HS, Kim SK, Lee J, Youm HW, Lee JR, Suh CS, et al. Effect of exogenous anti-Müllerian hormone treatment on cryopreserved and transplanted mouse ovaries. Reprod Sci. 2016; 23:51–60.
Article11. Lee JR, Youm HW, Kim SK, Jee BC, Suh CS, Kim SH. Effect of necrostatin on mouse ovarian cryopreservation and transplantation. Eur J Obstet Gynecol Reprod Biol. 2014; 178:16–20.
Article12. Seki S, Jin B, Mazur P. Extreme rapid warming yields high functional survivals of vitrified 8-cell mouse embryos even when suspended in a half-strength vitrification solution and cooled at moderate rates to -196°C. Cryobiology. 2014; 68:71–78.
Article13. Knight CA, Hallett J, DeVries AL. Solute effects on ice recrystallization: an assessment technique. Cryobiology. 1988; 25:55–60.
Article14. Lee HH, Lee HJ, Kim HJ, Lee JH, Ko Y, Kim SM, et al. Effects of antifreeze proteins on the vitrification of mouse oocytes: comparison of three different antifreeze proteins. Hum Reprod. 2015; 30:2110–2119.
Article15. Amir G, Rubinsky B, Basheer SY, Horowitz L, Jonathan L, Feinberg MS, et al. Improved viability and reduced apoptosis in subzero 21-hour preservation of transplanted rat hearts using antifreeze proteins. J Heart Lung Transplant. 2005; 24:1915–1929.
Article16. Peng L, Wang S, Yin S, Li C, Li Z, Wang S, et al. Autophosphorylation of H2AX in a cell-specific frozen dependent way. Cryobiology. 2008; 57:175–177.
Article17. Trapphoff T, El Hajj N, Zechner U, Haaf T, Eichenlaub-Ritter U. DNA integrity, growth pattern, spindle formation, chromosomal constitution and imprinting patterns of mouse oocytes from vitrified pre-antral follicles. Hum Reprod. 2010; 25:3025–3042.
Article18. Kobayashi J, Iwabuchi K, Miyagawa K, Sonoda E, Suzuki K, Takata M, et al. Current topics in DNA double-strand break repair. J Radiat Res. 2008; 49:93–103.
Article19. Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol. 2000; 10:886–895.
Article20. Tomczak MM, Hincha DK, Estrada SD, Feeney RE, Crowe JH. Antifreeze proteins differentially affect model membranes during freezing. Biochim Biophys Acta. 2001; 1511:255–263.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Antifreeze Protein on Mouse Ovarian Tissue Cryopreservation and Transplantation
- Effects of supplementation with antifreeze proteins on the follicular integrity of vitrified-warmed mouse ovaries: Comparison of two types of antifreeze proteins alone and in combination
- Influence of the vitrification solution on the angiogenic factors in vitrificated mouse ovarian tissue
- Ovarian Development of Vitrified Neonatal Ovaries after Orthotopic Transplantation into Adult Recipients
- Effect of Cryopreservation on the Heat Shock Protein 90 Expression in Mouse Ovarian Tissue