Intest Res.
2019 Apr;17(2):253-264. 10.5217/ir.2018.00062.
Development and validation of a scoring system for advanced colorectal neoplasm in young Korean subjects less than age 50 years
- Affiliations
-
- 1Division of Gastroenterology, Department of Internal Medicine, Mediplex Sejong Hospital, Incheon, Korea.
- 2The Research Institute of Basic Sciences, Seoul National University, Korea.
- 3Department of Statistics, Seoul National University, Seoul, Korea.
- 4Division of Gastroenterology, Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea. diksmc.park@samsung.com
- KMID: 2449967
- DOI: http://doi.org/10.5217/ir.2018.00062
Abstract
- BACKGROUND/AIMS
Colorectal cancer incidence among patients aged ≤50 years is increasing. This study aimed to develop and validate an advanced colorectal neoplasm (ACRN) screening model for young adults aged <50 years in Korea.
METHODS
This retrospective cross-sectional study included 59,575 consecutive asymptomatic Koreans who underwent screening colonoscopy between 2003 and 2012 at a single comprehensive health care center. Young Adult Colorectal Screening (YCS) score was developed as an optimized risk stratification model for ACRN using multivariate analysis and was internally validated. The predictive power and diagnostic performance of YCS score was compared with those of Asia-Pacific Colorectal Screening (APCS) and Korean Colorectal Screening (KCS) scores.
RESULTS
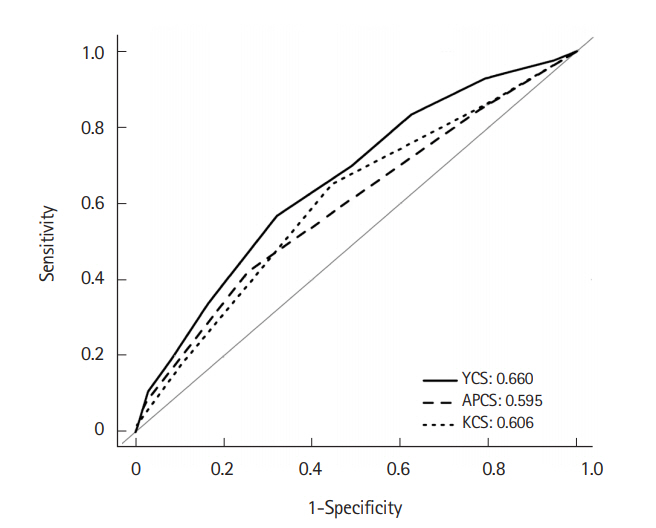
41,702 and 17,873 subjects were randomly allocated into the derivation and validation cohorts, respectively, by examination year. ACRN prevalence was 0.9% in both cohorts. YCS score comprised sex, age, alcohol, smoking, obesity, glucose metabolism abnormality, and family history of CRC, with score ranges of 0 to 10. In the validation cohort, ACRN prevalence was 0.6% in the low-risk tier (score, 0-4), 1.5% in the moderate-risk tier (score, 5-7), and 3.4% in the high-risk tier (score, 8-10). ACRN risk increased 2.5-fold (95% confidence interval [CI], 1.8-3.4) in the moderate-risk tier and 5.8-fold (95% CI, 3.4-9.8) in the high-risk tier compared with the low-risk tier. YCS score identified better balanced accuracy (53.9%) than APCS (51.5%) and KCS (50.7%) scores and had relatively good discriminative power (area under the curve=0.660).
CONCLUSIONS
YCS score based on clinical and laboratory risk factors was clinically effective and beneficial for predicting ACRN risk and targeting screening colonoscopy in adults aged <50 years.
MeSH Terms
Figure
Cited by 2 articles
-
How to Choose the Optimal Bowel Preparation Regimen for Colonoscopy
Ji Eun Na, Eun Ran Kim
Ewha Med J. 2021;44(4):122-132. doi: 10.12771/emj.2021.44.4.122.Prevalence and risk factors of colorectal cancer in Asia
Martin CS Wong, Hanyue Ding, Jingxuan Wang, Paul SF Chan, Junjie Huang
Intest Res. 2019;17(3):317-329. doi: 10.5217/ir.2019.00021.
Reference
-
1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–E386.
Article2. Jung KW, Won YJ, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat. 2015; 47:127–141.
Article3. St-Onge MP, Janssen I, Heymsfield SB. Metabolic syndrome in normal-weight Americans: new definition of the metabolically obese, normal-weight individual. Diabetes Care. 2004; 27:2222–2228.4. Winawer SJ, Zauber AG, O’Brien MJ, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps: the National Polyp Study Workgroup. N Engl J Med. 1993; 328:901–906.
Article5. Nishihara R, Wu K, Lochhead P, et al. Long-term colorectalcancer incidence and mortality after lower endoscopy. N Engl J Med. 2013; 369:1095–1105.
Article6. Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008; 134:1570–1595.
Article7. Sheng JQ, Li SR, Wu ZT, et al. Transferrin dipstick as a potential novel test for colon cancer screening: a comparative study with immuno fecal occult blood test. Cancer Epidemiol Biomarkers Prev. 2009; 18:2182–2185.
Article8. Freedman AN, Slattery ML, Ballard-Barbash R, et al. Colorectal cancer risk prediction tool for white men and women without known susceptibility. J Clin Oncol. 2009; 27:686–693.
Article9. Yeoh KG, Ho KY, Chiu HM, et al. The Asia-Pacific Colorectal Screening score: a validated tool that stratifies risk for colorectal advanced neoplasia in asymptomatic Asian subjects. Gut. 2011; 60:1236–1241.
Article10. Kim JK, Choi YS, Suh JP, Lee IT, Youk EG, Lee DS. Results of screening colonoscopy in asymptomatic average-risk Koreans at a community-based secondary hospital. Korean J Gastrointest Endosc. 2010; 41:266–272.11. Austin H, Henley SJ, King J, Richardson LC, Eheman C. Changes in colorectal cancer incidence rates in young and older adults in the United States: what does it tell us about screening. Cancer Causes Control. 2014; 25:191–201.
Article12. Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974-2013. J Natl Cancer Inst. 2017; 109.
Article13. World Health Organization, Regional Office for the Western Pacific; International Diabetes Institute; International Association for the Study of Obesity; International Obesity Task Force. The Asia-pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia;2000.14. Heitman SJ, Ronksley PE, Hilsden RJ, Manns BJ, Rostom A, Hemmelgarn BR. Prevalence of adenomas and colorectal cancer in average risk individuals: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2009; 7:1272–1278.
Article15. Kim DH, Cha JM, Shin HP, Joo KR, Lee JI, Park DI. Development and validation of a risk stratification-based screening model for predicting colorectal advanced neoplasia in Korea. J Clin Gastroenterol. 2015; 49:41–49.
Article16. Kahi CJ, Imperiale TF, Juliar BE, Rex DK. Effect of screening colonoscopy on colorectal cancer incidence and mortality. Clin Gastroenterol Hepatol. 2009; 7:770–775.
Article17. Rundle AG, Lebwohl B, Vogel R, Levine S, Neugut AI. Colonoscopic screening in average-risk individuals ages 40 to 49 vs 50 to 59 years. Gastroenterology. 2008; 134:1311–1315.
Article18. Sung JJ, Lau JY, Young GP, et al. Asia Pacific consensus recommendations for colorectal cancer screening. Gut. 2008; 57:1166–1176.
Article19. Chung SJ, Kim YS, Yang SY, et al. Prevalence and risk of colorectal adenoma in asymptomatic Koreans aged 40-49 years undergoing screening colonoscopy. J Gastroenterol Hepatol. 2010; 25:519–525.
Article20. Wong MC, Lam TY, Tsoi KK, et al. A validated tool to predict colorectal neoplasia and inform screening choice for asymptomatic subjects. Gut. 2014; 63:1130–1136.
Article21. Shin A, Hong CW, Sohn DK, et al. Associations of cigarette smoking and alcohol consumption with advanced or multiple colorectal adenoma risks: a colonoscopy-based case-control study in Korea. Am J Epidemiol. 2011; 174:552–562.
Article22. Regula J, Rupinski M, Kraszewska E, et al. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med. 2006; 355:1863–1872.
Article23. Tao S, Hoffmeister M, Brenner H. Development and validation of a scoring system to identify individuals at high risk for advanced colorectal neoplasms who should undergo colonoscopy screening. Clin Gastroenterol Hepatol. 2014; 12:478–485.
Article24. Park HW, Han S, Lee JS, et al. Risk stratification for advanced proximal colon neoplasm and individualized endoscopic screening for colorectal cancer by a risk-scoring model. Gastrointest Endosc. 2012; 76:818–828.
Article25. Cai QC, Yu ED, Xiao Y, et al. Derivation and validation of a prediction rule for estimating advanced colorectal neoplasm risk in average-risk Chinese. Am J Epidemiol. 2012; 175:584–593.
Article26. Ben Q, An W, Jiang Y, et al. Body mass index increases risk for colorectal adenomas based on meta-analysis. Gastroenterology. 2012; 142:762–772.
Article27. Jinjuvadia R, Lohia P, Jinjuvadia C, Montoya S, Liangpunsakul S. The association between metabolic syndrome and colorectal neoplasm: systemic review and meta-analysis. J Clin Gastroenterol. 2013; 47:33–44.28. Harriss DJ, Atkinson G, George K, et al. Lifestyle factors and colorectal cancer risk (1): systematic review and meta-analysis of associations with body mass index. Colorectal Dis. 2009; 11:547–563.
Article29. Kim KS, Moon HJ, Choi CH, et al. The frequency and risk factors of colorectal adenoma in health-check-up subjects in South Korea: relationship to abdominal obesity and age. Gut Liver. 2010; 4:36–42.
Article30. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004; 363:157–163.31. Gunter MJ, Leitzmann MF. Obesity and colorectal cancer: epidemiology, mechanisms and candidate genes. J Nutr Biochem. 2006; 17:145–156.
Article32. Eddi R, Karki A, Shah A, DeBari VA, DePasquale JR. Association of type 2 diabetes and colon adenomas. J Gastrointest Cancer. 2012; 43:87–92.
Article33. Singh S, Singh H, Singh PP, Murad MH, Limburg PJ. Antidiabetic medications and the risk of colorectal cancer in patients with diabetes mellitus: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2013; 22:2258–2268.
Article34. Hong SN, Kim JH, Choe WH, et al. Prevalence and risk of colorectal neoplasms in asymptomatic, average-risk screenees 40 to 49 years of age. Gastrointest Endosc. 2010; 72:480–489.
Article35. Kim JY, Jung YS, Park JH, et al. Different risk factors for advanced colorectal neoplasm in young adults. World J Gastroenterol. 2016; 22:3611–3620.
Article36. Choe JW, Chang HS, Yang SK, et al. Screening colonoscopy in asymptomatic average-risk Koreans: analysis in relation to age and sex. J Gastroenterol Hepatol. 2007; 22:1003–1008.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Strategies for colorectal cancer screening and post-polypectomy surveillance for young adults under age 50
- Is Colonoscopic Screening Necessary for Patients Younger than 50 Years with Gastric Adenoma or Cancer?
- Colon Cancer Screening—Is It Necessary to Start under the Age of 50?
- New Scoring System for Predicting Mortality in Patients with COVID-19
- Comparison of Colonoscopy Surveillance Outcomes Between Young and Older Colorectal Cancer Patients