J Liver Cancer.
2019 Mar;19(1):74-78. 10.17998/jlc.19.1.74.
A Case of Achieving Partial Remission with the Combination of Sorafenib and Nivolumab in a Patient with Hepatocellular Carcinoma Showing Disease Progression after Nivolumab Therapy
- Affiliations
-
- 1Department of Internal Medicine, Dongnam Institute of Radiological & Medical Sciences, Busan, Korea. mongmani@hanmail.net
- 2Department of Radiation Oncology, Dongnam Institute of Radiological & Medical Sciences, Busan, Korea.
- KMID: 2448281
- DOI: http://doi.org/10.17998/jlc.19.1.74
Abstract
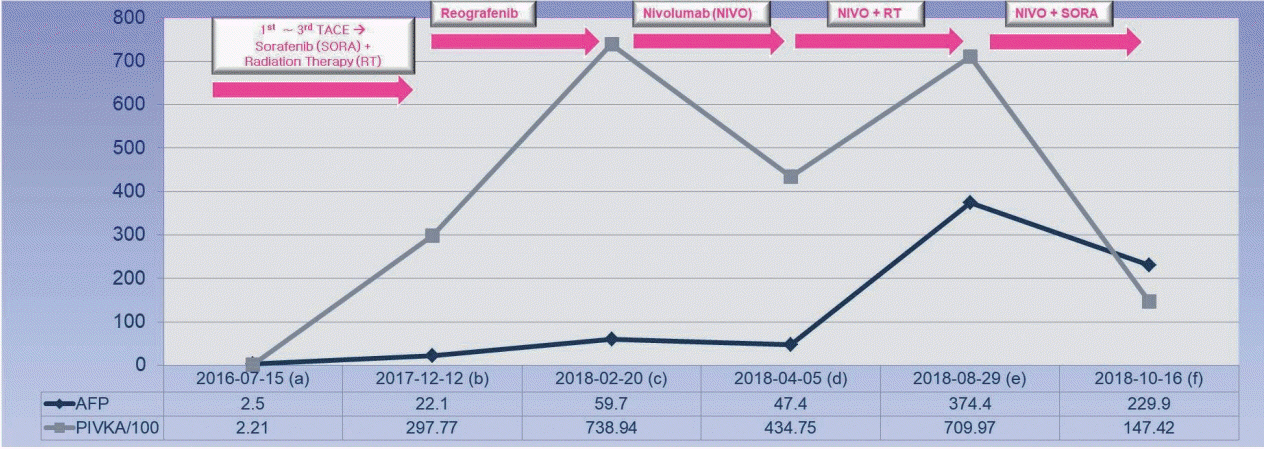
- Sorafenib is a well-known approved systemic therapeutic agent used in patients with advanced hepatocellular carcinoma (HCC). Regorafenib and nivolumab are approved as second-line therapeutic drugs in patients showing disease progression after sorafenib therapy. However, there is no established third- or fourth-line therapy in patients with progression after regorafenib or nivolumab treatment. Recently, the combination of tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICPIs) has been attempted as a first-line treatment strategy in advanced HCC patients based on the hypothesis that combination therapy may overcome resistance in ICPI monotherapy. On the basis of this suggestion, we herein describe the case of an HCC patient demonstrating macrovascular invasion, whereby partial remission was achieved via the combination of sorafenib and nivolumab following disease progression after nivolumab therapy. Further studies on the combination of TKIs and ICPIs are necessary to determine ways to manage HCC patients showing disease progression after ICPI therapy.
Keyword
MeSH Terms
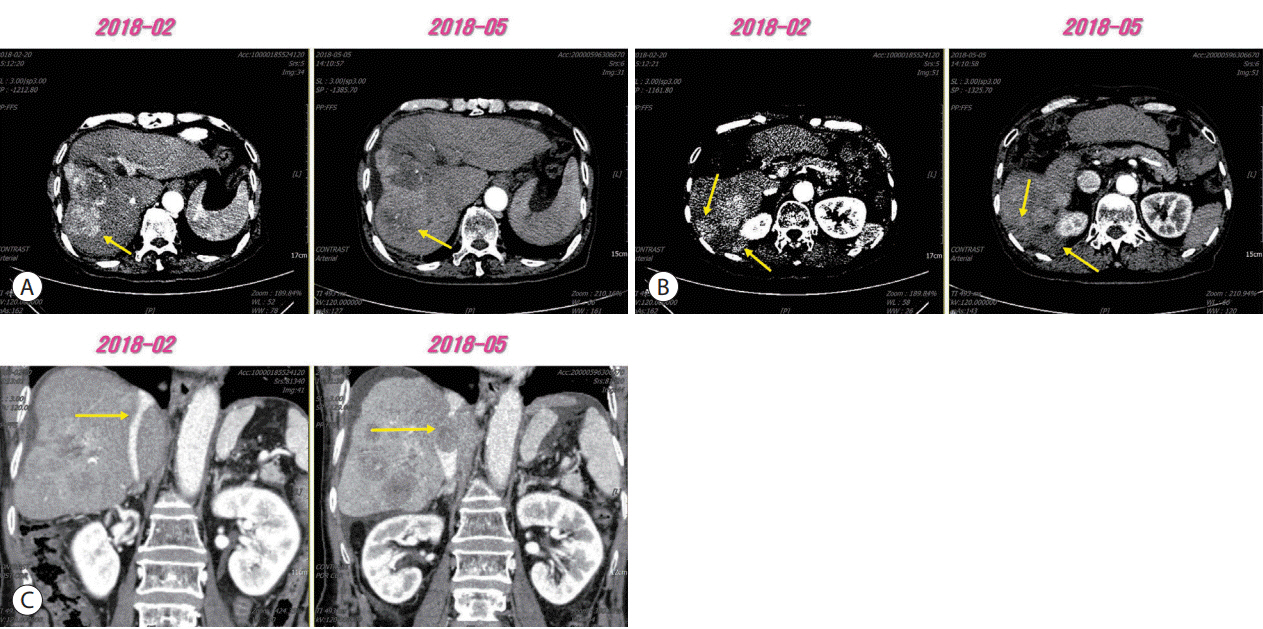
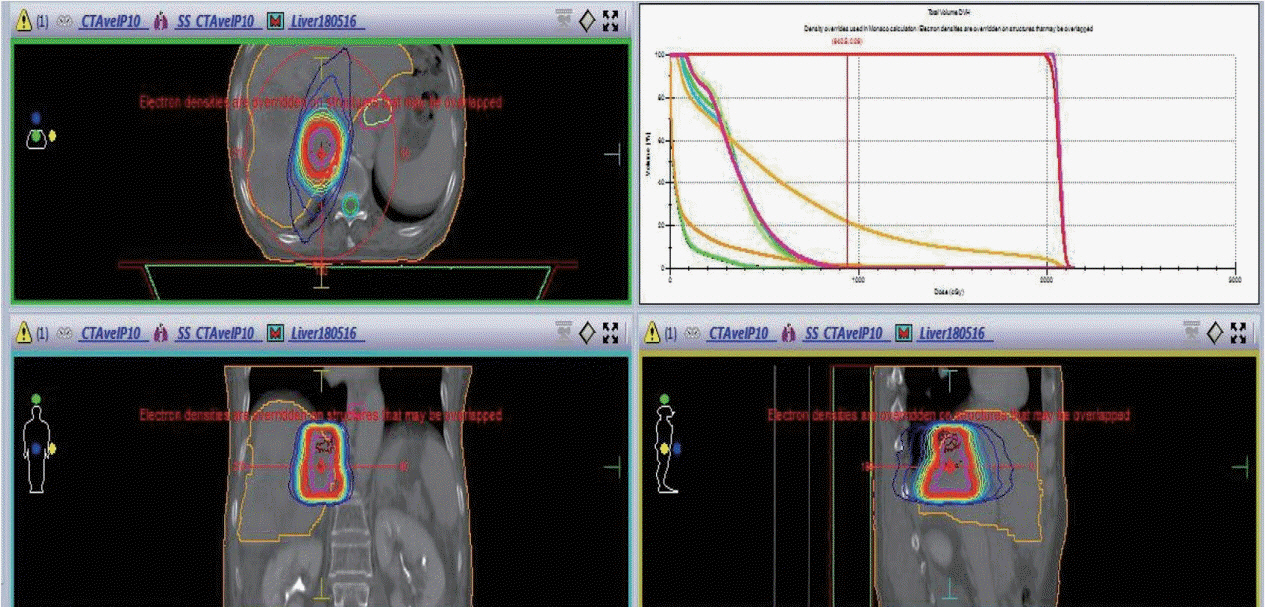
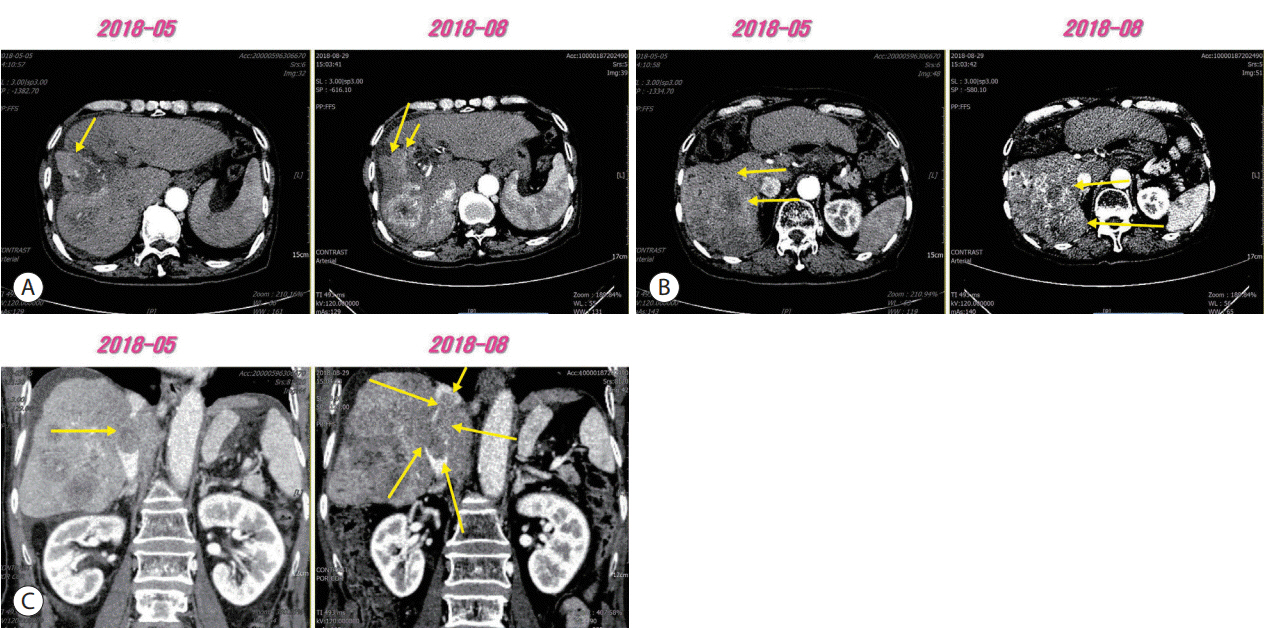
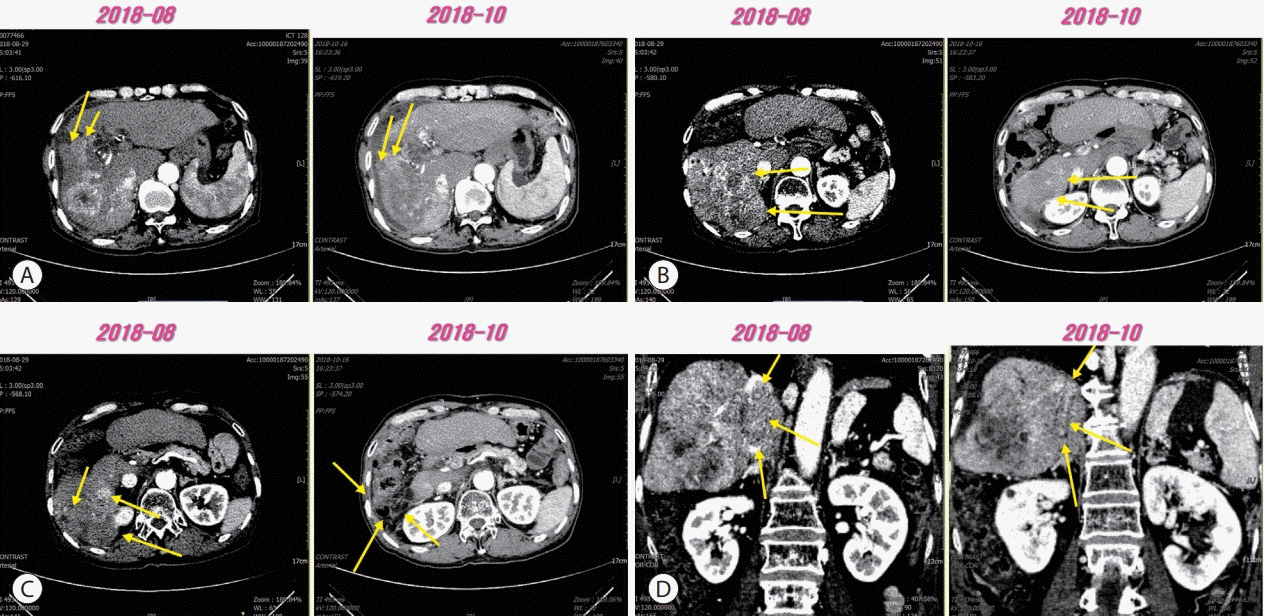
Figure
Reference
-
1. Cheng AL, Guan Z, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma according to baseline status: subset analyses of the phase III Sorafenib Asia-Pacific trial. Eur J Cancer. 2012; 48:1452–1465.2. Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L, Craxi A, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012; 57:821–829.3. Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, doubleblind, placebo-controlled, phase 3 trial. Lancet. 2017; 389:56–66.4. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the american association for the study of liver diseases. Hepatology. 2018; 68:723–750.5. Hughes PE, Caenepeel S, Wu LC. Targeted therapy and checkpoint immunotherapy combinations for the treatment of cancer. Trends Immunol. 2016; 37:462–476.6. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 noninferiority trial. Lancet. 2018; 391:1163–1173.7. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017; 389:2492–2502.8. Kudo M. Combination cancer immunotherapy in hepatoceullar carcinoma. Liver Cancer. 2018; 7:20–27.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Infiltrative hepatocellular carcinoma with multiple lung metastasis completely cured using nivolumab: a case report
- Nivolumab for Advanced Hepatocellular Carcinoma with Multiple Lung Metastases after Sorafenib Failure
- Sequential regorafenib or nivolumab therapy in recurrent hepatocellular carcinoma with sorafenib failure in liver transplant patients does not improve prognosis
- A Case of Achieving Complete Remission with Combination of Sorafenib and Tegafur in Patients with Hepatocellular Carcinoma with Progression of Disease after Sorafenib Therapy
- A case of nearly complete response in hepatocellular carcinoma with disseminated lung metastasis by combination therapy of nivolumab and ipilimumab after treatment failure of atezolizumab plus bevacizumab