Ann Dermatol.
2019 Jun;31(3):325-330. 10.5021/ad.2019.31.3.325.
Cowden Disease: Case Report and Review of the Literature
- Affiliations
-
- 1Department of Dermatology, Kangnam Sacred Heart Hospital, College of Medicine, Hallym University, Seoul, Korea. dermap@hanmail.net, hyeonekim@gmail.com
- KMID: 2444866
- DOI: http://doi.org/10.5021/ad.2019.31.3.325
Abstract
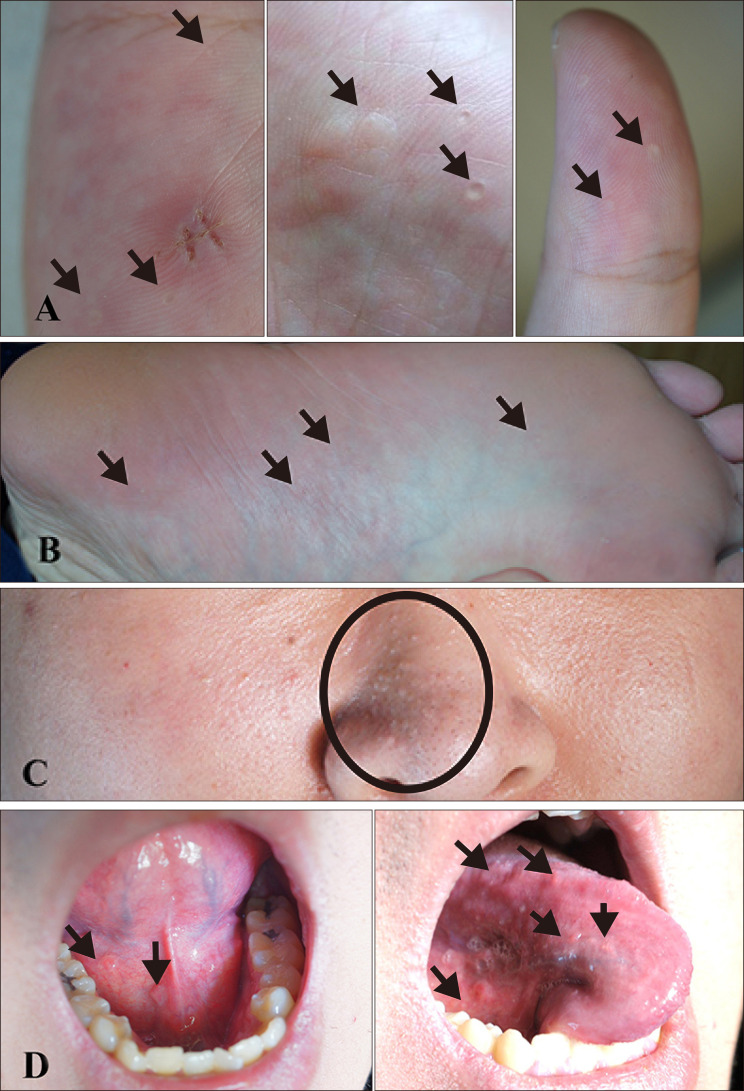
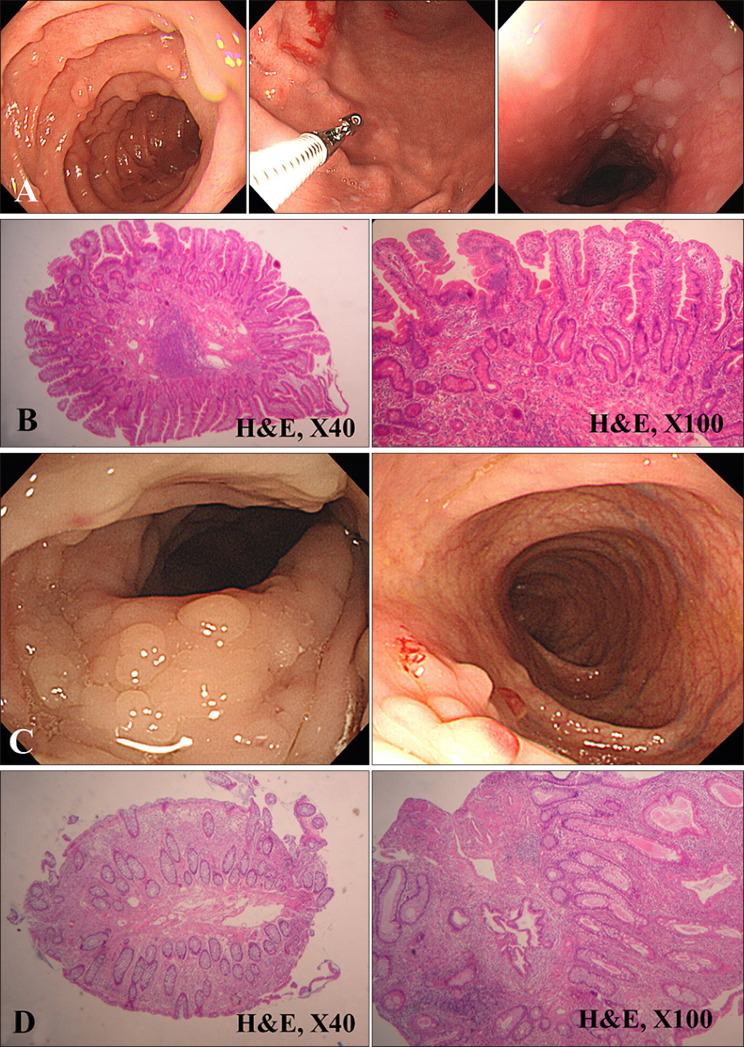
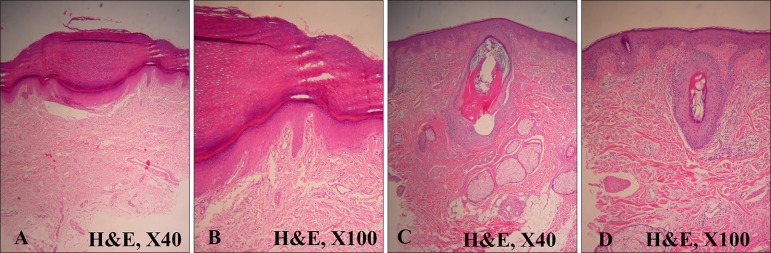
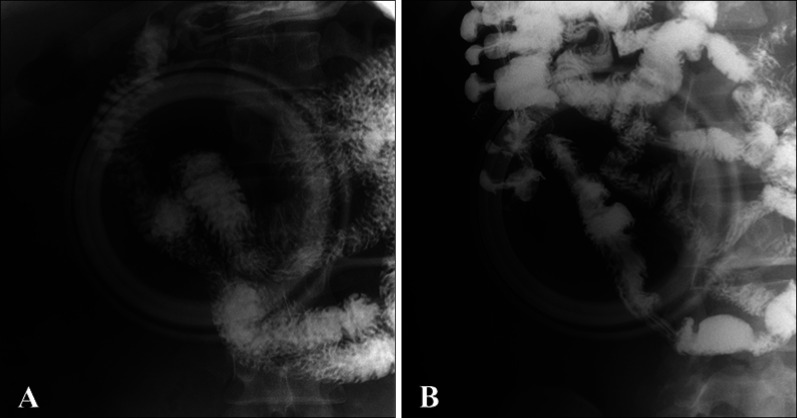
- Cowden's disease is a rare autosomal dominant, multiple hamartoma syndrome with characteristic mucocutaneous lesions. It is associated with abnormalities of the breast, thyroid, and gastrointestinal tract; and is characterized by multiple hamartomas in the gastrointestinal tract and mucocutaneous lesions such as trichilemmomas, oral papillomatosis, facial papules, and acral keratosis. A 21-year-old male patient presented with erythematous facial papules, oral mucosal papillomatosis, and punctate palmoplantar hyperkeratosis indicating a definite case of Cowden's disease. This disease derives from variable expression resulting from a mutation in the PTEN gene. Gastrointestinal endoscopy and colonoscopy revealed multiple hamartomas in the stomach and colon. On thyroid ultrasonography, several probable benign nodules were noted in the right thyroid gland. He had no pertinent family history and no other systemic findings. Further regular laboratory and image studies will be planned for our patient, as well as his family members. Sporadic Cowden's disease is rarely observed. Herein, we report a case of Cowden's disease without known family history. Dermatologists should be aware of the possibility of Cowden syndrome based on its several dermatologic findings.
MeSH Terms
Figure
Cited by 1 articles
-
Thyroid pathology, a clue to PTEN hamartoma tumor syndrome
Yurimi Lee, Young Lyun Oh
J Pathol Transl Med. 2023;57(3):178-183. doi: 10.4132/jptm.2023.03.04.
Reference
-
1. Seol JE, Park IH, Lee W, Kim H, Seo JK, Oh SH. Cowden syndrome with a novel germline PTEN mutation and an unusual clinical course. Ann Dermatol. 2015; 27:306–309. PMID: 26082588.2. Hong EJ, Kim HK, Cho YS, Ji JS, Kim CW, Kim CW, et al. A case of Cowden syndrome associated with breast cancer and thyroid cancer. Korean J Gastrointest Endosc. 2006; 32:293–297.3. Pilarski R, Burt R, Kohlman W, Pho L, Shannon KM, Swisher E. Cowden syndrome and the PTEN hamartoma tumor syndrome: systematic review and revised diagnostic criteria. J Natl Cancer Inst. 2013; 105:1607–1616. PMID: 24136893.
Article4. Lee HR, Moon YS, Yeom CH, Kim KW, Chun JY, Kim HK, et al. Cowden's disease--a report on the first case in Korea and literature review. J Korean Med Sci. 1997; 12:570–575. PMID: 9443100.
Article5. Bagan JV, Peñarrocha M, Vera-Sempere F. Cowden syndrome: clinical and pathological considerations in two new cases. J Oral Maxillofac Surg. 1989; 47:291–294. PMID: 2921660.
Article6. Ward SK, Roenigk HH, Gordon KB. Dermatologic manifestations of gastrointestinal disorders. Gastroenterol Clin North Am. 1998; 27:615–636. viPMID: 9891700.
Article7. Gustafson S, Zbuk KM, Scacheri C, Eng C. Cowden syndrome. Semin Oncol. 2007; 34:428–434. PMID: 17920899.
Article8. Salem OS, Steck WD. Cowden's disease (multiple hamartoma and neoplasia syndrome). A case report and review of the English literature. J Am Acad Dermatol. 1983; 8:686–696. PMID: 6863628.9. Eng C. Will the real Cowden syndrome please stand up: revised diagnostic criteria. J Med Genet. 2000; 37:828–830. PMID: 11073535.
Article10. Hildenbrand C, Burgdorf WH, Lautenschlager S. Cowden syndrome-diagnostic skin signs. Dermatology. 2001; 202:362–366. PMID: 11455162.11. Brownstein MH, Mehregan AH, Bikowski JB. Trichilemmomas in Cowden's disease. JAMA. 1977; 238:26.
Article12. Hong WK, Song HJ, Lee HS, Lee jR, Shin JH, Choi GS. A case of Cowden syndrome. Korean J Dermatol. 2007; 45:829–831.13. Nelen MR, Padberg GW, Peeters EA, Lin AY, van den Helm B, Frants RR, et al. Localization of the gene for Cowden disease to chromosome 10q22-23. Nat Genet. 1996; 13:114–116. PMID: 8673088.
Article14. Celebi JT, Shendrik I, Silvers DN, Peacocke M. Identification of PTEN mutations in metastatic melanoma specimens. J Med Genet. 2000; 37:653–657. PMID: 10978354.
Article15. Stewart AL, Mhashilkar AM, Yang XH, Ekmekcioglu S, Saito Y, Sieger K, et al. PI3 kinase blockade by Ad-PTEN inhibits invasion and induces apoptosis in RGP and metastatic melanoma cells. Mol Med. 2002; 8:451–461. PMID: 12435856.16. Butler MG, Dasouki MJ, Zhou XP, Talebizadeh Z, Brown M, Takahashi TN, et al. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J Med Genet. 2005; 42:318–321. PMID: 15805158.
Article17. Varga EA, Pastore M, Prior T, Herman GE, McBride KL. The prevalence of PTEN mutations in a clinical pediatric cohort with autism spectrum disorders, developmental delay, and macrocephaly. Genet Med. 2009; 11:111–117. PMID: 19265751.
Article18. Danielsen SA, Lind GE, Bjørnslett M, Meling GI, Rognum TO, Heim S, et al. Novel mutations of the suppressor gene PTEN in colorectal carcinomas stratified by microsatellite instability- and TP53 mutation- status. Hum Mutat. 2008; 29:E252–E262. PMID: 18781614.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cowden's disease--a report on the first case in Korea and literature review
- Breast Cancer in Cowden Syndrome: A Case Report
- A case of Cowden's syndrome associated with Lhermitte-Duclos disease
- Cowden Syndrome: A Review
- Multiorgan Involvements of Cowden Disease in a 50-Year-Old Woman: A Case Report and Literature Overview