J Korean Ophthalmol Soc.
2019 Apr;60(4):362-368. 10.3341/jkos.2019.60.4.362.
Analysis of Intraocular Pressure Elevation after Intravitreal Injection of Ranibizumab and Aflibercept
- Affiliations
-
- 1Department of Ophthalmology, Chonnam National University Medical School, Gwangju, Korea. exo70@naver.com
- KMID: 2443153
- DOI: http://doi.org/10.3341/jkos.2019.60.4.362
Abstract
- PURPOSE
To evaluate long-term intraocular pressure (IOP) and risk of IOP elevation after intravitreal injection of ranibizumab or aflibercept in patients with age-related macular degeneration (AMD).
METHODS
From January 2013 to December 2016, we retrospectively reviewed patients who underwent intravitreal ranibizumab or aflibercept injections for AMD. IOP was measured before injection and 1 week, 1 month, 2 months, 3 months, 4 months, 5 months, 6 months, 9 months, and 1 year after injection. Sustained IOP elevation was defined when the final IOP increased by 6 mmHg more than the pre-injection IOP, and when there were two consecutively measured values > 21 mmHg. The risk factors were then analyzed.
RESULTS
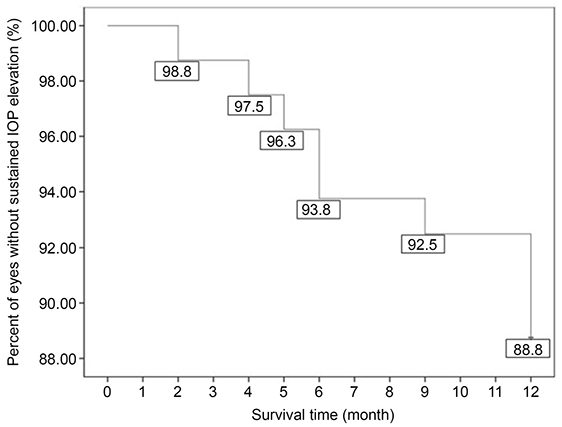
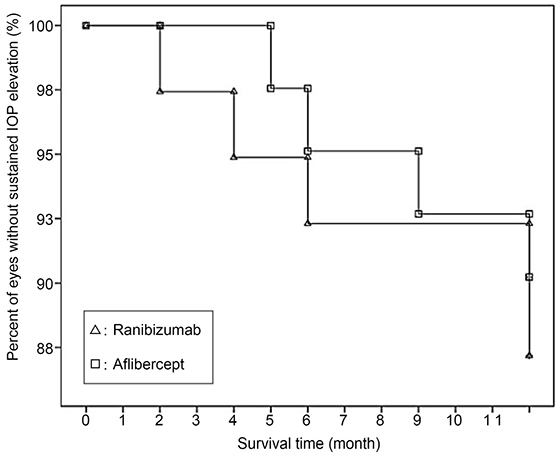
Using Kaplan-Meier survival analysis, sustained IOP elevation occurred in 9 of 80 eyes (11.3%) in 1 year, and the mean survival time was 11.50 months after injection. Five eyes (12.8%) of the ranibizumab group and four eyes (9.8%) of the aflibercept group had mean survival times of 11.39 and 11.61 months, respectively. The log-rank test showed no significant difference between the two groups (p = 0.659). A significant risk factor for sustained IOP elevation was a history of primary open-angle glaucoma (p = 0.035).
CONCLUSIONS
The incidence of sustained IOP elevation was not significantly different between the two groups. Clinicians should therefore carefully monitor the IOP before and after intravitreal ranibizumab or aflibercept injections, especially in AMD patients with primary open-angle glaucoma.
MeSH Terms
Figure
Reference
-
1. Freund KB, Hoang QV, Saroj N, Thompson D. Intraocular pressure in patients with neovascular age-related macular degeneration receiving intravitreal aflibercept or ranibizumab. Ophthalmology. 2015; 122:1802–1810.
Article2. Lee G, Lee S. Full-thickness macular hole after intravitreal aflibercept injection in a patient with wet age-related macular degeneration. J Korean Ophthalmol Soc. 2017; 58:875–878.
Article3. Dedania VS, Bakri SJ. Sustained elevation of intraocular pressure after intravitreal anti-VEGF agents: what is the evidence? Retina. 2015; 35:841–858.4. Hoang QV, Mendonca LS, Della Torre KE, et al. Effect on intraocular pressure in patients receiving unilateral intravitreal anti-vascular endothelial growth factor injections. Ophthalmology. 2012; 119:321–326.
Article5. Menke MN, Salam A, Framme C, Wolf S. Long-term intraocular pressure changes in patient with neovascular age-related macular degeneration with ranibizumab. Ophthalmologica. 2013; 229:168–172.6. Tseng JJ, Vance SK, Della Torre KE, et al. Sustained increased intraocular pressure related to intravitreal antivascular endothelial growth factor therapy for neovascular age-related macular degeneration. J Glaucoma. 2012; 21:241–247.
Article7. Pershing S, Bakri SJ, Moshfeghi DM. Ocular hypertension and intraocular pressure asymmetry after intravitreal injection of antivascular endothelial growth factor agents. Ophthalmic Surg Lasers Imaging Retina. 2013; 44:460–464.
Article8. Min JS, Jung HC, Suh JY, Kwon YH. Comparison between aflibercept, ranibizumab intravitreal injection on neovascular age-related macular degeneration patients. J Korean Ophthalmol Soc. 2016; 57:1738–1744.
Article9. Bracha P, Moore NA, Ciulla TA, et al. The acute and chronic effects of intravitreal anti-vascular endothelial growth factor injections on intraocular pressure: a review. Surv Ophthalmol. 2018; 63:281–295.
Article10. Drance SM. The significance of the diurnal tension variations in normal and glaucomatous eyes. Arch Ophthalmol. 1960; 64:494–501.
Article11. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–1431.
Article12. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1432–1444.
Article13. Rusu IM, Deobhakta A, Yoon D, et al. Intraocular pressure in patients with neovascular age-related macular degeneration switched to aflibercept injection after injection after previous anti-VEGF treatments. Retina. 2014; 34:2161–2166.14. Good TJ, Kimura AE, Mandava N, Kahook MY. Sustained elevation of intraocular pressure after intravitreal injections of anti-VEGF agents. Br J Ophthalmol. 2011; 95:1111–1114.
Article15. Hoang QV, Tsuang AJ, Gelman R, et al. Clinical predictors of sustained intraocular pressure elevation due to intravitreal antivascular endothelial growth factor therapy. Retina. 2013; 33:179–187.
Article16. Bakri SJ, McCannel CA, Edwards AO, Moshfeghi DM. Persistent ocular hypertension following intravitreal ranibizumab. Graefes Arch Clin Exp Ophthalmol. 2008; 246:955–958.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Full-thickness Macular Hole after Intravitreal Aflibercept Injection in a Patient with Wet Age-related Macular Degeneration
- A Multimodal Approach to Diabetic Macular Edema
- Comparison of Short-Term Clinical Outcomes between Intravitreal Ranibizumab and Aflibercept in Retinal Angiomatous Proliferation
- Intravitreal Ranibizumab Injection in Adult-onset Coats' Disease: A Case Report
- Efficacy of Primary Intravitreal Ranibizumab Injection for Treatment of Type 1 Retinopathy of Prematurity