Nat Prod Sci.
2019 Mar;25(1):76-80. 10.20307/nps.2019.25.1.76.
Nitric Oxide Inhibitory Constituents from the Fruits of Amomum tsao-ko
- Affiliations
-
- 1College of Pharmacy, Chungbuk National University, Cheongju 28160, Republic of Korea. byhwang@chungbuk.ac.kr
- KMID: 2443108
- DOI: http://doi.org/10.20307/nps.2019.25.1.76
Abstract
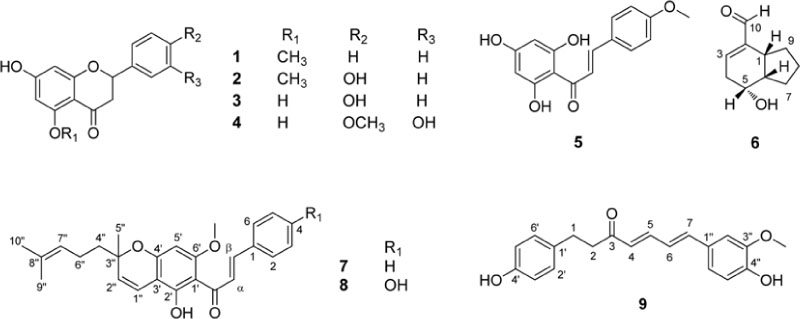
- Bioactivity-guided fractionation of MeOH extract of the dried fruits of Amomum tsao-ko led to isolation of nine compounds (1 - 9). Their structures were elucidated by spectroscopic methods including extensive 1D and 2D-NMR, as alpinetin (1), naringenin-5-O-methyl ether (2), naringenin (3), hesperetin (4), 2"²,4"²,6"²-trihydroxy-4-methoxy chalcone (5), tsaokoin (6), boesenbergin B (7), 4-hydroxyboesenbergin B (8), and tsaokoarylone (9). Of these, compound 8 was isolated from a natural source for the first time, which was previously reported as a synthetic product. The isolated compounds (1 - 9) were tested for their inhibitory effects on LPS-induced nitric oxide production in RAW 264.7 macrophages. Among them, three chalcone derivatives (compounds 5, 7, and 8) and a diarylheptanoid (compound 9) exhibited significant inhibitory activity on the NO production with ICâ‚…â‚€ values ranging from 10.9 to 22.5 µM.
Figure
Reference
-
1. Tang W, Eisenbrand B. Handbook of Chinese medicinal plants: Chemistry, pharmacology, toxicology. Weinheim: Wiley-VCH;2011. p. 106.2. Yang X, Küenzi P, Plitzko I, Potterat O, Hamburger M. Planta Med. 2009; 75:543–546.3. Lee KY, Kim SH, Sung SH, Kim YC. Planta Med. 2008; 74:867–869.4. Starkenmann C, Mayenzet F, Brauchli R, Wunsche L, Vial C. J Agric Food Chem. 2007; 55:10902–10907.5. Hong SS, Lee JH, Choi YH, Jeong W, Ahn EK, Lym SH, Oh JS. Tetrahedron Lett. 2015; 56:6681–6684.6. Itokawa H, Morita M, Mihashi S. Phytochemistry. 1981; 20:2503–2506.7. Hammami S, Ben Jannet H, Bergaoui A, Ciavatta L, Cimino G, Mighri Z. Molecules. 2004; 9:602–608.8. Jeon SH, Chun W, Choi YJ, Kwon YS. Arch Pharm Res. 2008; 31:978–982.9. Ibrahim AR, Galal AM, Ahmed MS, Mossa GS. Chem Pharm Bull (Tokyo). 2003; 51:203–206.10. McCormick S, Robson K, Bohm B. Phytochemistry. 1985; 24:1614–1616.11. Moon SS, Lee JY, Cho SC. J Nat Prod. 2004; 67:889–891.12. Win NN, Awale S, Esumi H, Tezuka Y, Kadota S. J Nat Prod. 2007; 70:1582–1587.13. L Aigner E Oberbauer-Hofmann S Couillard-Despres FJ Rivera H Riepl C Urmann M Biendl . US patents. 9527860B2. 2016.14. Moon SS, Cho SC, Lee JY. Bull Korean Chem Soc. 2005; 26:447–450.15. Mariotto S, Suzuki Y, Persichini T, Colasanti M, Suzuki H, Cantoni O. Curr Med Chem. 2007; 14:1940–1944.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bioassay-coupled LC-QTOF MS/MS to Characterize Constituents Inhibiting Nitric Oxide Production of Thuja orientalis
- Role of nitric oxide and distribution of nitric oxide synthase in the gustatory system
- Dose-Dependent Inhibitory Effect of Nitric Oxide on Embryo Development
- Local Delivery of Nitric Oxide Donor
- Nitric Oxide: The Pathophysiological Roles and Clinical Implications in Circulatory System