Dement Neurocogn Disord.
2016 Sep;15(3):75-81. 10.12779/dnd.2016.15.3.75.
Is Parkinson's Disease with History of Agent Orange Exposure Different from Idiopathic Parkinson's Disease?
- Affiliations
-
- 1Department of Neurology, Veteran Health Service Medical Center, Seoul, Korea.
- 2Department of Nuclear Medicine, Veteran Health Service Medical Center, Seoul, Korea.
- 3Department of Neurology, Hyoja Geriatric Hospital, Yongin, Korea. kwakdr@gmail.com
- KMID: 2442849
- DOI: http://doi.org/10.12779/dnd.2016.15.3.75
Abstract
- BACKGROUND AND PURPOSE
During Vietnam War, many Korean soldiers were dispatched to fight in the war where they were exposed to Agent Orange. Until now, there exist only limited evidence on existence of association between exposure to Agent Orange and Parkinson's disease (PD). To elucidate the effects of Agent Orange exposure on PD, we compared the clinical characteristics and radiolabeled 18F-FP-CIT PET uptake between patients with Agent Orange exposure and patients with Agent Orange no-exposure.
METHODS
We retrospectively evaluated 143 patients exposed to Agent Orange and 500 patients with no exposure to Agent Orange from our movement clinics database. The differences between clinical characteristics and pattern of 18F-FP-CIT PET uptake were investigated.
RESULTS
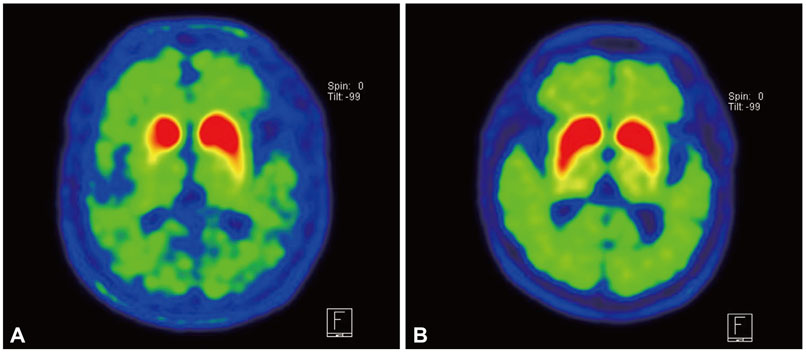
Among Unified Parkinson's Disease Rating Scale III motor subscales, tremor at rest, rigidity, finger taps, and rapid alternating movement was significantly higher in patients exposed to Agent Orange as compared to patients with no exposure to Agent Orange. The facial expression score was significantly lower in patients exposed to Agent Orange as compared to patients with no exposure to Agent Orange. Compared to patients not exposed to Agent Orange, all basal ganglia areas (contra- and ipsilateral caudate nucleus, anterior putamen, and posterior putamen) showed a lower18F-FP-CIT uptake and higher asymmetry index of anterior and posterior putamen was found in patients exposed to Agent Orange. The caudate/putamen ratio was significantly lower in patients exposed to Agent Orange as compared to patients with no exposure to Agent Orange.
CONCLUSIONS
This study showed a different clinical profile and FP-CIT PET findings between patients exposed to Agent Orange as compared to patients with no exposure to Agent Orange. This finding suggests the possibility of different pathophysiology of PD in patients exposed to Agent Orange from idiopathic PD.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Plasma Oligomeric Beta Amyloid in Alzheimer's Disease with History of Agent Orange Exposure
YoungSoon Yang, Vo Van Giau, Seong Soo A. An, SangYun Kim
Dement Neurocogn Disord. 2018;17(2):41-49. doi: 10.12779/dnd.2018.17.2.41.
Reference
-
1. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992; 55:181–184.
Article2. Decouflé P, Holmgreen P, Boyle CA, Stroup NE. Self-reported health status of Vietnam veterans in relation to perceived exposure to herbicides and combat. Am J Epidemiol. 1992; 135:312–323.
Article3. Lewis SJ, Foltynie T, Blackwell AD, Robbins TW, Owen AM, Barker RA. Heterogeneity of Parkinson's disease in the early clinical stages using a data driven approach. J Neurol Neurosurg Psychiatry. 2005; 76:343–348.
Article4. Isaias IU, Benti R, Cilia R, Canesi M, Marotta G, Gerundini P, et al. [123I]FP-CIT striatal binding in early Parkinson's disease patients with tremor vs. akinetic-rigid onset. Neuroreport. 2007; 18:1499–1502.
Article5. Zober A, Ott MG, Messerer P. Morbidity follow up study of BASF employees exposed to 2,3,7, 8-tetrachlorodibenzo-p-dioxin (TCDD) after a 1953 chemical reactor incident. Occup Environ Med. 1994; 51:479–486.
Article6. Peper M, Klett M, Frentzel-Beyme R, Heller WD. Neuropsychological effects of chronic exposure to environmental dioxins and furans. Environ Res. 1993; 60:124–135.
Article7. Neuberger M, Rappe C, Bergek S, Cai H, Hansson M, Jäger R, et al. Persistent health effects of dioxin contamination in herbicide production. Environ Res. 1999; 81:206–214.
Article8. Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003; 39:889–909.9. van der Mark M, Brouwer M, Kromhout H, Nijssen P, Huss A, Vermeulen R. Is pesticide use related to Parkinson disease? Some clues to heterogeneity in study results. Environ Health Perspect. 2012; 120:340–347.
Article10. Konjuh C, García G, López L, de Duffard AM, Brusco A, Duffard R. Neonatal hypomyelination by the herbicide 2,4-dichlorophenoxyacetic acid. Chemical and ultrastructural studies in rats. Toxicol Sci. 2008; 104:332–340.
Article11. Williamson MA, Gasiewicz TA, Opanashuk LA. Aryl hydrocarbon receptor expression and activity in cerebellar granule neuroblasts: implications for development and dioxin neurotoxicity. Toxicol Sci. 2005; 83:340–348.
Article12. Sarnico I, Boroni F, Benarese M, Sigala S, Lanzillotta A, Battistin L, et al. Activation of NF-kappaB p65/c-Rel dimer is associated with neuroprotection elicited by mGlu5 receptor agonists against MPP(+) toxicity in SK-N-SH cells. J Neural Transm (Vienna). 2008; 115:669–676.
Article13. Filbrandt CR, Wu Z, Zlokovic B, Opanashuk L, Gasiewicz TA. Presence and functional activity of the aryl hydrocarbon receptor in isolated murine cerebral vascular endothelial cells and astrocytes. Neurotoxicology. 2004; 25:605–616.
Article14. Bongiovanni B, Ferri A, Brusco A, Rassetto M, Lopez LM, Evangelista de Duffard AM, et al. Adverse effects of 2,4-dichlorophenoxyacetic acid on rat cerebellar granule cell cultures were attenuated by amphetamine. Neurotox Res. 2011; 19:544–555.
Article15. Bortolozzi AA, Evangelista De Duffard AM, Duffard RO, Antonelli MC. Effects of 2,4-dichlorophenoxyacetic acid exposure on dopamine D2-like receptors in rat brain. Neurotoxicol Teratol. 2004; 26:599–605.
Article16. Bortolozzi A, Evangelista de Duffard AM, Dajas F, Duffard R, Silveira R. Intracerebral administration of 2,4-diclorophenoxyacetic acid induces behavioral and neurochemical alterations in the rat brain. Neurotoxicology. 2001; 22:221–232.
Article17. Hely MA, Morris JG, Reid WG, O'Sullivan DJ, Williamson PM, Broe GA, et al. Age at onset: the major determinant of outcome in Parkinson's disease. Acta Neurol Scand. 1995; 92:455–463.
Article18. Gómez-Esteban JC, Tijero B, Ciordia R, Berganzo K, Somme J, Lezcano E, et al. Factors influencing the symmetry of Parkinson's disease symptoms. Clin Neurol Neurosurg. 2010; 112:302–305.
Article19. Uc EY, McDermott MP, Marder KS, Anderson SW, Litvan I, Como PG, et al. Incidence of and risk factors for cognitive impairment in an early Parkinson disease clinical trial cohort. Neurology. 2009; 73:1469–1477.
Article20. Moratalla R, Quinn B, DeLanney LE, Irwin I, Langston JW, Graybiel AM. Differential vulnerability of primate caudate-putamen and striosome-matrix dopamine systems to the neurotoxic effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc Natl Acad Sci U S A. 1992; 89:3859–3863.
Article21. Marek KL, Seibyl JP, Zoghbi SS, Zea-Ponce Y, Baldwin RM, Fussell B, et al. [123I] beta-CIT/SPECT imaging demonstrates bilateral loss of dopamine transporters in hemi-Parkinson's disease. Neurology. 1996; 46:231–237.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Plasma Oligomeric Beta Amyloid in Alzheimer's Disease with History of Agent Orange Exposure
- Diagnosis and Treatment of Parkinson's Disease
- Urinary Dysfunction in Idiopathic Parkinson's Disease
- Idiopathic Parkinson's Disease Presenting with Sleep Terrors
- The History of Parkinson's Disease and Famous Patients