Transl Clin Pharmacol.
2019 Mar;27(1):19-23. 10.12793/tcp.2019.27.1.19.
Evolving role of modeling and simulation in drug development
- Affiliations
-
- 1Department of Clinical Pharmacology and Therapeutics, Asan Medical Center, University of Ulsan, Seoul 05505, Republic of Korea. mdhslim@gmail.com
- KMID: 2442242
- DOI: http://doi.org/10.12793/tcp.2019.27.1.19
Abstract
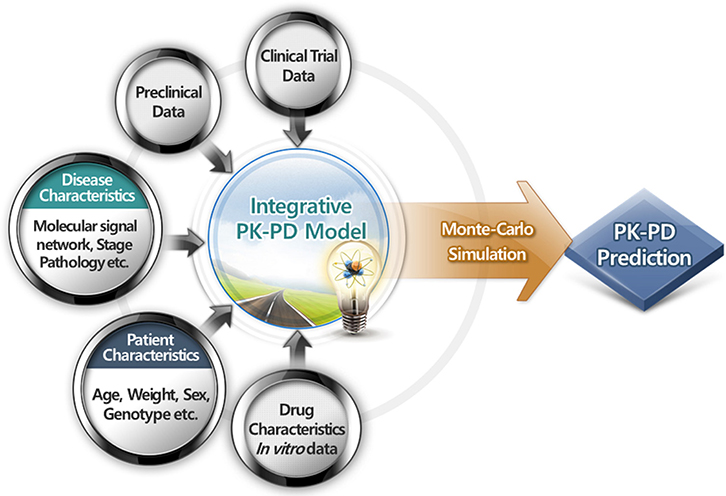
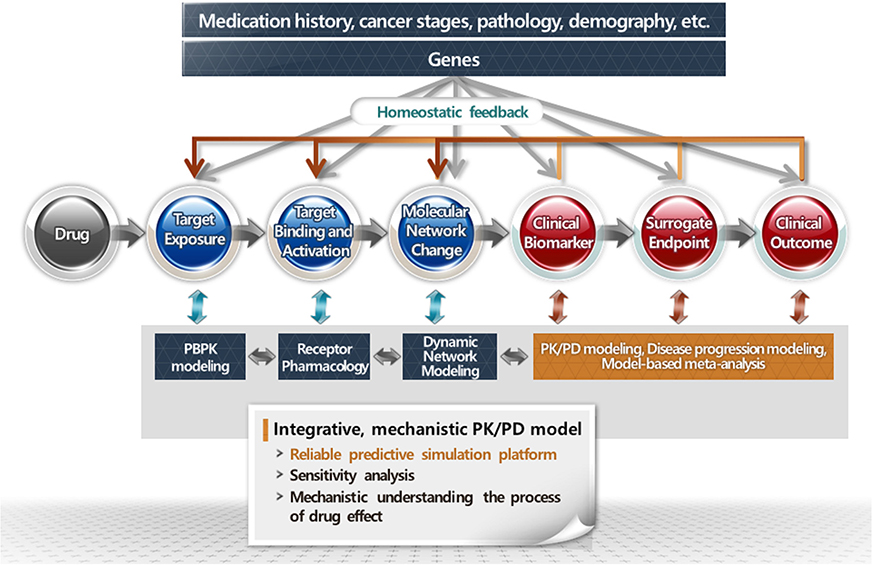
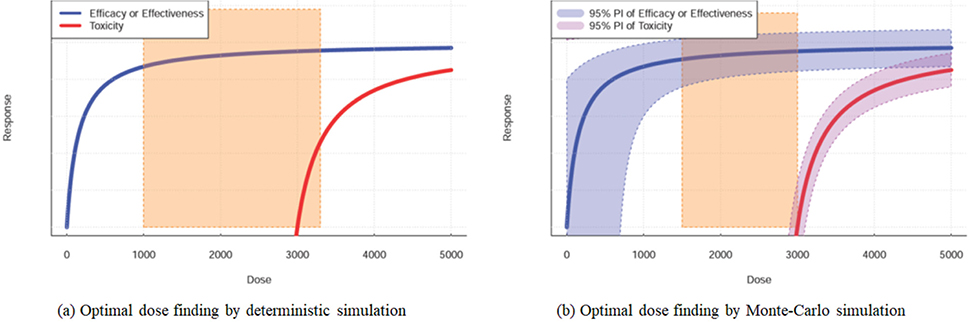
- Pharmacokinetic-pharmacodynamic model is a kind of language that quantitatively describes the drug-related outcomes in the form of mathematical formula. Various outcomes can be subjected to modeling analysis if they can be expressed in numbers. Empirical models have been widely and successfully applied in drug development and research. However, a more competitive drug development environment requires more accurate and predictive models in the early stages of drug development. Accordingly, the subjects of PK-PD modeling have been extended from clinical data to preclinical and in vitro data in the discovery stage. More mechanistic and predictive models, such as physiologically based pharmacokinetic and quantitative system-based pharmacology models, are being increasingly used owing to the growing need to characterize drugs more accurately at the earliest. This tutorial briefly introduces the essential concepts of PK-PD modeling and simulation and describes the recent changing roles of PK-PD model for application in novel drug development process.
MeSH Terms
Figure
Reference
-
1. Milligan PA, Brown MJ, Marchant B, Martin SW, van der Graaf PH, Benson N, et al. Model-based drug development: a rational approach to efficiently accelerate drug development. Clin Pharmacol Ther. 2013; 93:502–514. DOI: 10.1038/clpt.2013.54.
Article2. Arrowsmith J. Trial watch: Phase II failures: 2008-2010. Nat Rev Drug Discov. 2011; 10:328–329. DOI: 10.1038/nrd3439.3. Arrowsmith J. Trial watch: phase III and submission failures: 2007-2010. Nat Rev Drug Discov. 2011; 10:87. DOI: 10.1038/nrd3375.4. Food and Drug Administration. Critical path opportunities report. Accessed 20 February 2018. http://www.fda.gov/ScienceResearch/SpecialTopics/CriticalPathInitiative.5. Zhuang X, Lu C. PBPK modeling and simulation in drug research and development. Acta Pharm Sin B. 2016; 6:430–440.
Article6. Rowland M, Peck C, Tucker G. Physiologically-based pharmacokinetics in drug development and regulatory science. Annu Rev Pharmacol Toxicol. 2011; 51:45–73. DOI: 10.1146/annurev-pharmtox-010510-100540.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A review of three years' experience of the first pharmacometrics company in Korea
- Prediction of pharmacokinetics and drug-drug interaction potential using physiologically based pharmacokinetic (PBPK) modeling approach: A case study of caffeine and ciprofloxacin
- Computational modeling of atrial fibrillation
- A Review of Modeling Approaches to Predict Drug Response in Clinical Oncology
- Protocol Analysis for Expert System of Nurses Scheduling