Korean J Physiol Pharmacol.
2017 Jan;21(1):107-115. 10.4196/kjpp.2017.21.1.107.
Prediction of pharmacokinetics and drug-drug interaction potential using physiologically based pharmacokinetic (PBPK) modeling approach: A case study of caffeine and ciprofloxacin
- Affiliations
-
- 1College of Pharmacy, Chungnam National University, Daejeon 34134, Korea. yshin@cnu.ac.kr
- 2Department of Chemistry and Research Institute for Basic Sciences, Kyung Hee University, Seoul 02453, Korea.
- 3Advanced Analysis Center, Korea Institute of Science and Technology, Seoul 02792, Korea.
- KMID: 2371074
- DOI: http://doi.org/10.4196/kjpp.2017.21.1.107
Abstract
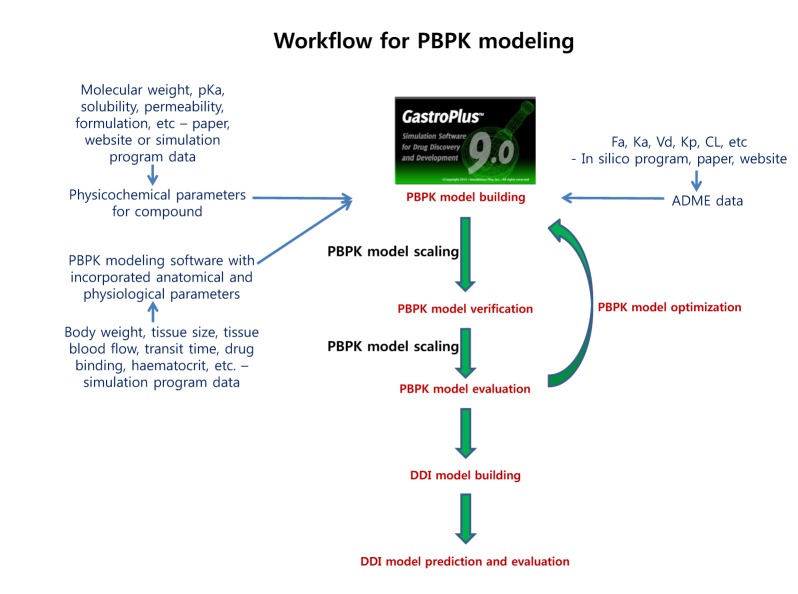
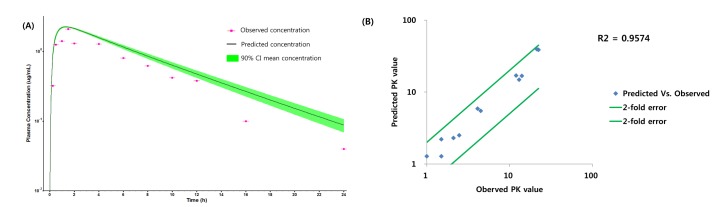
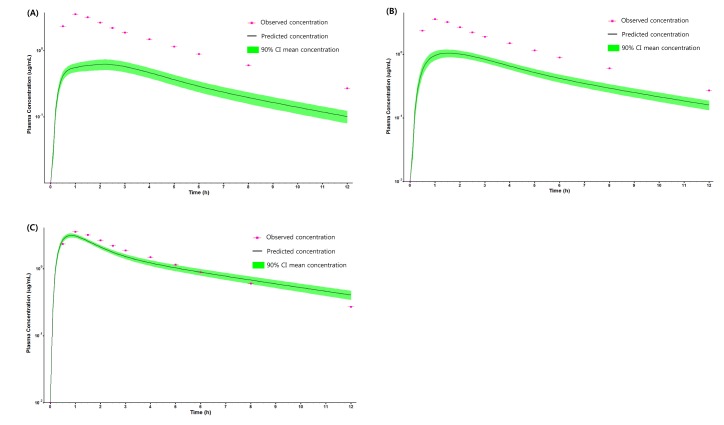
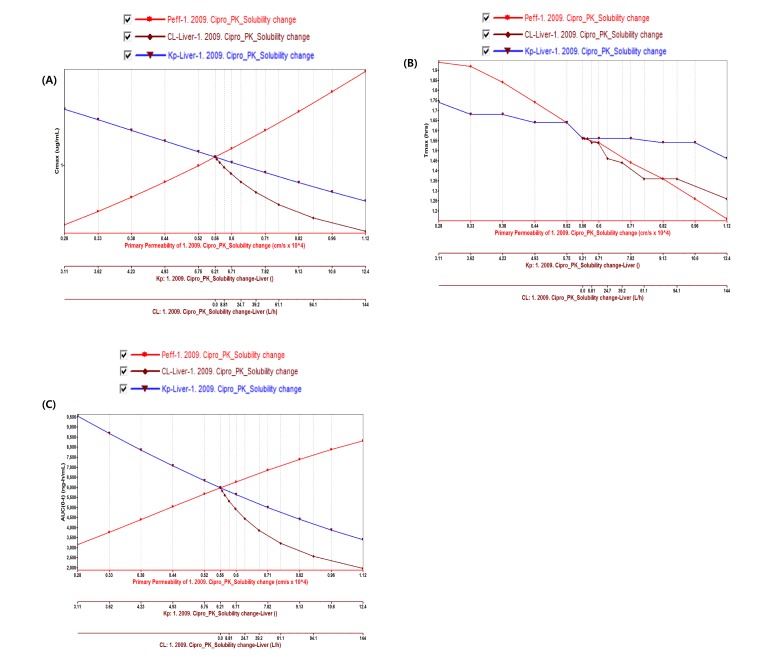
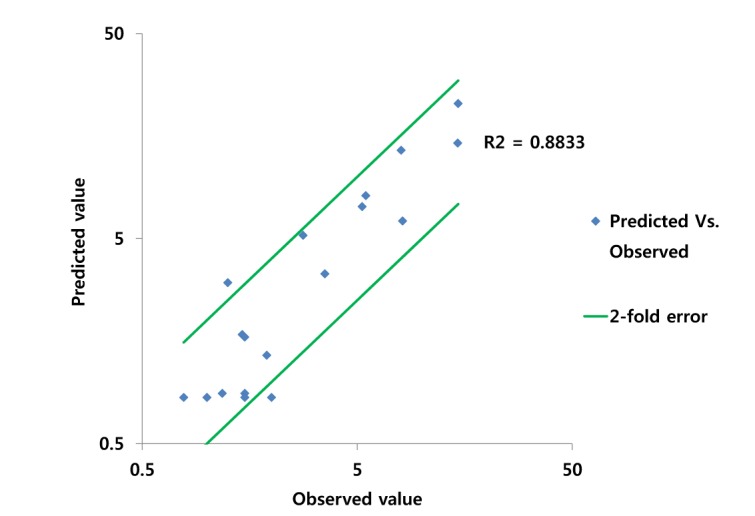
- Over the last decade, physiologically based pharmacokinetics (PBPK) application has been extended significantly not only to predicting preclinical/human PK but also to evaluating the drug-drug interaction (DDI) liability at the drug discovery or development stage. Herein, we describe a case study to illustrate the use of PBPK approach in predicting human PK as well as DDI using in silico, in vivo and in vitro derived parameters. This case was composed of five steps such as: simulation, verification, understanding of parameter sensitivity, optimization of the parameter and final evaluation. Caffeine and ciprofloxacin were used as tool compounds to demonstrate the "fit for purpose" application of PBPK modeling and simulation for this study. Compared to caffeine, the PBPK modeling for ciprofloxacin was challenging due to several factors including solubility, permeability, clearance and tissue distribution etc. Therefore, intensive parameter sensitivity analysis (PSA) was conducted to optimize the PBPK model for ciprofloxacin. Overall, the increase in C(max) of caffeine by ciprofloxacin was not significant. However, the increase in AUC was observed and was proportional to the administered dose of ciprofloxacin. The predicted DDI and PK results were comparable to observed clinical data published in the literatures. This approach would be helpful in identifying potential key factors that could lead to significant impact on PBPK modeling and simulation for challenging compounds.
MeSH Terms
Figure
Reference
-
1. Hosea NA, Jones HM. Predicting pharmacokinetic profiles using in silico derived parameters. Mol Pharm. 2013; 10:1207–1215. PMID: 23427934.
Article2. Krayenbühl JC, Vozeh S, Kondo-Oestreicher M, Dayer P. Drug-drug interactions of new active substances: mibefradil example. Eur J Clin Pharmacol. 1999; 55:559–565. PMID: 10541773.
Article3. Bjornsson TD, Callaghan JT, Einolf HJ, Fischer V, Gan L, Grimm S, Kao J, King SP, Miwa G, Ni L, Kumar G, McLeod J, Obach SR, Roberts S, Roe A, Shah A, Snikeris F, Sullivan JT, Tweedie D, Vega JM, Walsh J, Wrighton SA. Pharmaceutical Research and Manufacturers of America Drug Metabolism/Clinical Pharmacology Technical Working Groups. The conduct of in vitro and in vivo drug-drug interaction studies: a PhRMA perspective. J Clin Pharmacol. 2003; 43:443–469. PMID: 12751267.
Article4. Einolf HJ. Comparison of different approaches to predict metabolic drug-drug interactions. Xenobiotica. 2007; 37:1257–1294. PMID: 17968745.
Article5. Almond LM, Yang J, Jamei M, Tucker GT, Rostami-Hodjegan A. Towards a quantitative framework for the prediction of DDIs arising from cytochrome P450 induction. Curr Drug Metab. 2009; 10:420–432. PMID: 19519348.
Article6. Kato M, Chiba K, Horikawa M, Sugiyama Y. The quantitative prediction of in vivo enzyme-induction caused by drug exposure from in vitro information on human hepatocytes. Drug Metab Pharmacokinet. 2005; 20:236–243. PMID: 16141603.
Article7. Ripp SL, Mills JB, Fahmi OA, Trevena KA, Liras JL, Maurer TS, de Morais SM. Use of immortalized human hepatocytes to predict the magnitude of clinical drug-drug interactions caused by CYP3A4 induction. Drug Metab Dispos. 2006; 34:1742–1748. PMID: 16837568.
Article8. Shou M, Hayashi M, Pan Y, Xu Y, Morrissey K, Xu L, Skiles GL. Modeling, prediction, and in vitro in vivo correlation of CYP3A4 induction. Drug Metab Dispos. 2008; 36:2355–2370. PMID: 18669588.
Article9. Fahmi OA, Maurer TS, Kish M, Cardenas E, Boldt S, Nettleton D. A combined model for predicting CYP3A4 clinical net drug-drug interaction based on CYP3A4 inhibition, inactivation, and induction determined in vitro. Drug Metab Dispos. 2008; 36:1698–1708. PMID: 18490437.
Article10. Kozawa M, Honma M, Suzuki H. Quantitative prediction of in vivo profiles of CYP3A4 induction in humans from in vitro results with a reporter gene assay. Drug Metab Dispos. 2009; 37:1234–1241. PMID: 19282397.
Article11. Guo H, Liu C, Li J, Zhang M, Hu M, Xu P, Liu L, Liu X. A mechanistic physiologically based pharmacokinetic-enzyme turnover model involving both intestine and liver to predict CYP3A induction-mediated drug-drug interactions. J Pharm Sci. 2013; 102:2819–2836. PMID: 23760985.
Article12. Baneyx G, Fukushima Y, Parrott N. Use of physiologically based pharmacokinetic modeling for assessment of drug-drug interactions. Future Med Chem. 2012; 4:681–693. PMID: 22458685.
Article13. Zhao P, Zhang L, Grillo JA, Liu Q, Bullock JM, Moon YJ, Song P, Brar SS, Madabushi R, Wu TC, Booth BP, Rahman NA, Reynolds KS, Gil Berglund E, Lesko LJ, Huang SM. Applications of physiologically based pharmacokinetic (PBPK) modeling and simulation during regulatory review. Clin Pharmacol Ther. 2011; 89:259–267. PMID: 21191381.
Article14. Chen Y, Jin JY, Mukadam S, Malhi V, Kenny JR. Application of IVIVE and PBPK modeling in prospective prediction of clinical pharmacokinetics: strategy and approach during the drug discovery phase with four case studies. Biopharm Drug Dispos. 2012; 33:85–98. PMID: 22228214.
Article15. Jones HM, Dickins M, Youdim K, Gosset JR, Attkins NJ, Hay TL, Gurrell IK, Logan YR, Bungay PJ, Jones BC, Gardner IB. Application of PBPK modelling in drug discovery and development at Pfizer. Xenobiotica. 2012; 42:94–106. PMID: 22035569.
Article16. Yamazaki S, Skaptason J, Romero D, Vekich S, Jones HM, Tan W, Wilner KD, Koudriakova T. Prediction of oral pharmacokinetics of cMet kinase inhibitors in humans: physiologically based pharmacokinetic model versus traditional one-compartment model. Drug Metab Dispos. 2011; 39:383–393. PMID: 21098644.
Article17. Bungay PJ, Tweedy S, Howe DC, Gibson KR, Jones HM, Mount NM. Preclinical and clinical pharmacokinetics of PF-02413873, a nonsteroidal progesterone receptor antagonist. Drug Metab Dispos. 2011; 39:1396–1405. PMID: 21543556.
Article18. Jones HM, Gardner IB, Watson KJ. Modelling and PBPK simulation in drug discovery. AAPS J. 2009; 11:155–166. PMID: 19280352.
Article19. Yu LX, Amidon GL. A compartmental absorption and transit model for estimating oral drug absorption. Int J Pharm. 1999; 186:119–125. PMID: 10486429.
Article20. Sawada Y, Hanano M, Sugiyama Y, Harashima H, Iga T. Prediction of the volumes of distribution of basic drugs in humans based on data from animals. J Pharmacokinet Biopharm. 1984; 12:587–596. PMID: 6533294.
Article21. Poulin P, Theil FP. A priori prediction of tissue:plasma partition coefficients of drugs to facilitate the use of physiologically-based pharmacokinetic models in drug discovery. J Pharm Sci. 2000; 89:16–35. PMID: 10664535.
Article22. Heikkinen AT, Baneyx G, Caruso A, Parrott N. Application of PBPK modeling to predict human intestinal metabolism of CYP3A substrates - an evaluation and case study using GastroPlus. Eur J Pharm Sci. 2012; 47:375–386. PMID: 22759901.
Article23. Zhang T, Heimbach T, Lin W, Zhang J, He H. Prospective predictions of human pharmacokinetics for eighteen compounds. J Pharm Sci. 2015; 104:2795–2806. PMID: 25690565.
Article24. Baneyx G, Parrott N, Meille C, Iliadis A, Lavé T. Physiologically based pharmacokinetic modeling of CYP3A4 induction by rifampicin in human: influence of time between substrate and inducer administration. Eur J Pharm Sci. 2014; 56:1–15. PMID: 24530864.
Article25. Staib AH, Stille W, Dietlein G, Shah PM, Harder S, Mieke S, Beer C. Interaction between quinolones and caffeine. Drugs. 1987; 34(Suppl 1):170–174.
Article26. Harder S, Staib AH, Beer C, Papenburg A, Stille W, Shah PM. 4-quinolones inhibit biotransformation of caffeine. Eur J Clin Pharmacol. 1988; 35:651–656. PMID: 2853056.
Article27. Healy DP, Polk RE, Kanawati L, Rock DT, Mooney ML. Interaction between oral ciprofloxacin and caffeine in normal volunteers. Antimicrob Agents Chemother. 1989; 33:474–478. PMID: 2729942.
Article28. Zhang L, Wei MJ, Zhao CY, Qi HM. Determination of the inhibitory potential of 6 fluoroquinolones on CYP1A2 and CYP2C9 in human liver microsomes. Acta Pharmacol Sin. 2008; 29:1507–1514. PMID: 19026171.
Article29. Valizadeh H, Hamishehkar H, Ghanbarzadeh S, Zabihian N, Zakeri-Milani P. Pharmacokinetics and bioequivalence evaluation of two brands of ciprofloxacin 500 mg tablets in Iranian healthy volunteers. Arzneimittelforschung. 2012; 62:566–570. PMID: 23047470.30. Boxenbaum H. Interspecies pharmacokinetic scaling and the evolutionary-comparative paradigm. Drug Metab Rev. 1984; 15:1071–1121. PMID: 6396053.
Article31. Jones HM, Gardner IB, Collard WT, Stanley PJ, Oxley P, Hosea NA, Plowchalk D, Gernhardt S, Lin J, Dickins M, Rahavendran SR, Jones BC, Watson KJ, Pertinez H, Kumar V, Cole S. Simulation of human intravenous and oral pharmacokinetics of 21 diverse compounds using physiologically based pharmacokinetic modelling. Clin Pharmacokinet. 2011; 50:331–347. PMID: 21456633.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Physiologically-based pharmacokinetic modeling of nafamostat to support dose selection for treatment of pediatric patients with COVID-19
- Qualification and application of liquid chromatography-quadrupole time-offlight mass spectrometric method for the determination of carisbamate in rat plasma and prediction of its human pharmacokinetics using physiologically based pharmacokinetic modeling
- Compatibility Study between Physiologically Based Pharmacokinetic (PBPK) and Compartmental PK Model Using Lumping Method: Application to the Voriconazole Case
- Predicting the systemic exposure and lung concentration of nafamostat using physiologically-based pharmacokinetic modeling
- Physiologically-based pharmacokinetic model for clozapine in Korean patients with schizophrenia