Ann Rehabil Med.
2019 Feb;43(1):81-86. 10.5535/arm.2019.43.1.81.
Diagnostic Significance of Fibrin Degradation Products and D-Dimer in Patients With Breast Cancer-Related Lymphedema
- Affiliations
-
- 1Department of Physical Medicine and Rehabilitation, Kosin University Gospel Hospital, Busan, Korea. oggum@hanmail.net
- 2Department of Rehabilitation Medicine, Konkuk University School of Medicine, Chungju, Korea.
- KMID: 2440955
- DOI: http://doi.org/10.5535/arm.2019.43.1.81
Abstract
OBJECTIVE
To find out whether levels of fibrin degradation products (FDP) and D-dimer are increased in breast cancer-related lymphedema (BCRL) as in many vascular diseases. FDP and D-dimer have been used in blood tests to help differentiate deep vein thrombosis in the diagnosis of lymphedema. Levels of FDP and D-dimer are often elevated in patients with BCRL.
METHODS
Patients with BCRL (group I), non-lymphedema after breast cancer treatment (group II), and deep venous thrombosis (group III) from January 2012 to December 2016 were enrolled. Levels of FDP and D-dimer were measured in all groups and compared among groups.
RESULTS
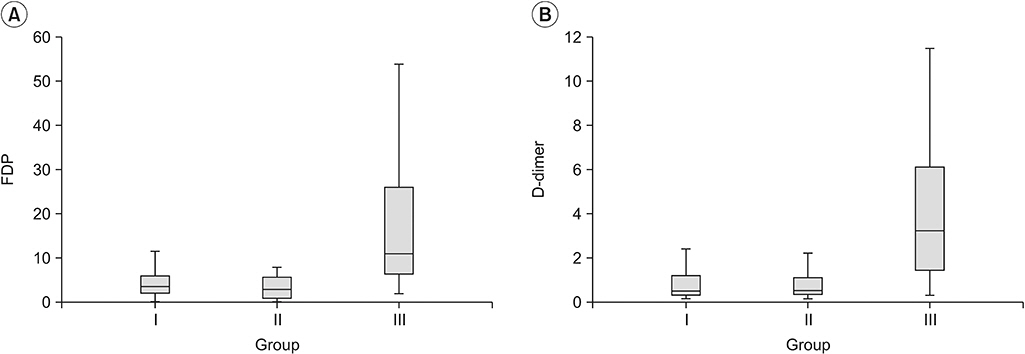
Mean values of FDP and D-dimer of group I were 5.614±12.387 and 1.179±2.408 μg/μL, respectively. These were significantly higher than their upper normal limits set in our institution. Levels of FDP or D-dimer were not significantly different between group I and group II. However, values of FDP and D-dimer in group III were significantly higher than those in group I.
CONCLUSION
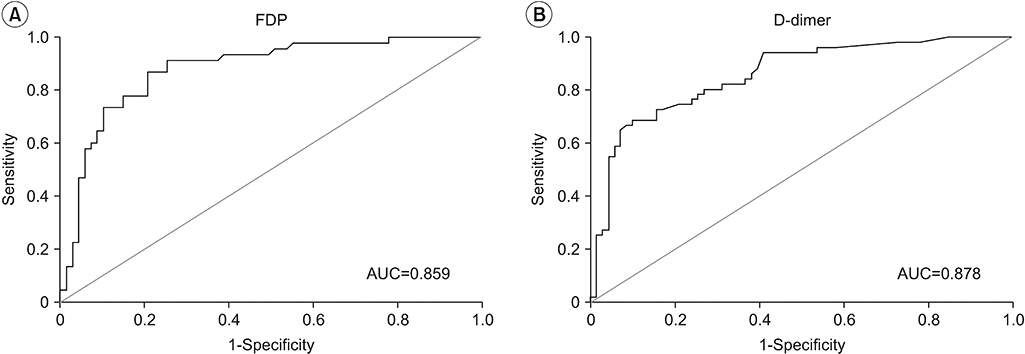
Values of FDP and D-dimer were much higher in patients with thrombotic disease than those in patients with lymphedema. Thus, FDP and D-dimer can be used to differentiate between DVT and lymphedema. However, elevated levels of FDP or D-dimer cannot indicate the occurrence of lymphedema.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Effects of Different Bandaging Methods for Treating Patients With Breast Cancer-Related Lymphedema
Se Hyun Oh, Sung Hwan Ryu, Ho Joong Jeong, Jung Hyun Lee, Young-Joo Sim
Ann Rehabil Med. 2019;43(6):677-685. doi: 10.5535/arm.2019.43.6.677.
Reference
-
1. Maclellan RA, Greene AK. Lymphedema. Semin Pediatr Surg. 2014; 23:191–7.
Article2. Rockson SG. Lymphedema. Am J Med. 2001; 110:288–95.
Article3. Yang GH, Shim JY. The diagnosis and treatment of lymphedema. J Korean Med Assoc. 2013; 56:1115–22.
Article4. Tripodi A. D-dimer testing in laboratory practice. Clin Chem. 2011; 57:1256–62.
Article5. Heil J, Miesbach W, Vogl T, Bechstein WO, Reinisch A. Deep vein thrombosis of the upper extremity. Dtsch Arztebl Int. 2017; 114:244–9.
Article6. Joffe HV, Kucher N, Tapson VF, Goldhaber SZ; Deep Vein Thrombosis (DVT) FREE Steering Committee. Upper-extremity deep vein thrombosis: a prospective registry of 592 patients. Circulation. 2004; 110:1605–11.
Article7. Munoz FJ, Mismetti P, Poggio R, Valle R, Barron M, Guil M, et al. Clinical outcome of patients with upperextremity deep vein thrombosis: results from the RIETE Registry. Chest. 2008; 133:143–8.8. Wong P, Baglin T. Epidemiology, risk factors and sequelae of venous thromboembolism. Phlebology. 2012; 27 Suppl 2:2–11.
Article9. Martinelli I, Bucciarelli P, Mannucci PM. Thrombotic risk factors: basic pathophysiology. Crit Care Med. 2010; 38(2 Suppl):S3–9.
Article10. Lasinski BB, McKillip Thrift K, Squire D, Austin MK, Smith KM, et al. A systematic review of the evidence for complete decongestive therapy in the treatment of lymphedema from 2004 to 2011. PM R. 2012; 4:580–601.
Article11. Bounameaux H, Schneider PA, Reber G, de Moerloose P, Krahenbuhl B. Measurement of plasma D-dimer for diagnosis of deep venous thrombosis. Am J Clin Pathol. 1989; 91:82–5.
Article12. Minari C, Cecconami L, Fioravanti A, Montemerani M, Scola C, Marcolongo R. Lymphoedema of the limbs in rheumatoid arthritis. Clin Rheumatol. 1994; 13:464–9.
Article13. Moskovitz AH, Anderson BO, Yeung RS, Byrd DR, Lawton TJ, Moe RE. Axillary web syndrome after axillary dissection. Am J Surg. 2001; 181:434–9.
Article14. Lippi G, Favaloro EJ, Cervellin G. Hemostatic properties of the lymph: relationships with occlusion and thrombosis. Semin Thromb Hemost. 2012; 38:213–21.
Article15. Brinkhous KM, Walker SA. Prothrombin and fibrinogen in lymph. Am J Physiol. 1941; 132:666–9.
Article16. Adam SS, Key NS, Greenberg CS. D-dimer antigen: current concepts and future prospects. Blood. 2009; 113:2878–87.
Article17. Kabrhel C, Mark Courtney D, Camargo CA Jr, Plewa MC, Nordenholz KE, Moore CL, et al. Factors associated with positive D-dimer results in patients evaluated for pulmonary embolism. Acad Emerg Med. 2010; 17:589–97.
Article18. Gardiner C, Pennaneac’h C, Mackie IJ, Sheldrake A, Harrison J, Machin SJ. Falsely elevated D-dimer results in a healthy patient on account of heterophiletul antibodies. Br J Haematol. 2003; 122:871–3.
Article19. Tobbia D, Semple J, Baker A, Dumont D, Semple A, Johnston M. Lymphedema development and lymphatic function following lymph node excision in sheep. J Vasc Res. 2009; 46:426–34.
Article20. Guc E, Briquez PS, Foretay D, Fankhauser MA, Hubbell JA, Kilarski WW, et al. Local induction of lymphangiogenesis with engineered fibrin-binding VEGFC promotes wound healing by increasing immune cell trafficking and matrix remodeling. Biomaterials. 2017; 131:160–75.
Article21. Kim C, Li B, Papaiconomou C, Zakharov A, Johnston M. Functional impact of lymphangiogenesis on fluid transport after lymph node excision. Lymphology. 2003; 36:111–9.22. Zampell JC, Avraham T, Yoder N, Fort N, Yan A, Weitman ES, et al. Lymphatic function is regulated by a coordinated expression of lymphangiogenic and antilymphangiogenic cytokines. Am J Physiol Cell Physiol. 2012; 302:C392–404.
Article23. Welsh JD, Kahn ML, Sweet DT. Lymphovenous hemostasis and the role of platelets in regulating lymphatic flow and lymphatic vessel maturation. Blood. 2016; 128:1169–73.
Article24. McColl BK, Baldwin ME, Roufail S, Freeman C, Moritz RL, Simpson RJ, et al. Plasmin activates the lymphangiogenic growth factors VEGF-C and VEGF-D. J Exp Med. 2003; 198:863–8.
Article25. Bui HM, Enis D, Robciuc MR, Nurmi HJ, Cohen J, Chen M, et al. Proteolytic activation defines distinct lymphangiogenic mechanisms for VEGFC and VEGFD. J Clin Invest. 2016; 126:2167–80.
Article26. de Abreu Junior GF, Pitta GB, Araujo M, Castro Ade A, de Azevedo Junior WF, Miranda Junior F. Ultrasonografic changes in the axillary vein of patients with lymphedema after mastectomy. Rev Col Bras Cir. 2015; 42:81–92.27. Baarslag HJ, van Beek EJ, Koopman MM, Reekers JA. Prospective study of color duplex ultrasonography compared with contrast venography in patients suspected of having deep venous thrombosis of the upper extremities. Ann Intern Med. 2002; 136:865–72.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comments on Point of Care D-Dimer Testing in the Emergency Department: A Bioequivalence Study
- D-Dimer Testing in Laboratory Practice
- Plasma Levels of D-dimer and Fibrinogen/Fibrin Degradation Products According to Subtypes of Ischemic Stroke
- Response to the Comments on 'Point of Care D-Dimer Testing in the Emergency Department-A Bioequivalence Study' and Erratum to the Results
- Diagnostic Usefulness of a Relative Increase in the Ratio Between D-dimer and C-reactive Protein in Pulmonary Thromboembolism Disorder